If we don’t start holding people accountable by the 2024 (s)elections, nobody will ever be held accountable. Every single candidate MUST be pressed as to their position on scrapping the injection program altogether. Anyone not willing to take that stand, or anyone not will to even answer the question, is complicit in these ongoing crimes against humanity. You must not reward three years of treachery, coming from most members of both parties at every level of government, with your vote if they will not even address the past three years.
- jw
#DoNotComply #NoCompromise #NoSurrender #GallowAndGuillotines2023
Source: The Daily Clout
Report 76: Pfizer Had Data to Announce Its COVID-19 Vaccine’s Alleged “Efficacy” in October 2020. Why Did Pfizer Delay the Announcement Until November 9, 2020, Six Days After the 2020 U.S. Presidential Election?
July 10, 2023 • by Jeyanthi Kunadhasan, MD, FANZCA, and Ed Clark, MSE – Team 3
Executive summary
On July 21, 2020, the United States (U.S.) government, Pfizer, and BioNTech signed a contract, known as a Statement of Work (SOW), for the rapid development of and obtaining regulatory licensure for a COVID-19 vaccine by October 31, 2020, a few days prior to the November 3, 2020, U.S. Presidential election. Regulatory licensure was to be based on approval by the U.S. Food and Drug Administration (FDA) or authorization based on demonstration of efficacy. (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf)
The July 2020 SOW states, “…if FDA approval or authorization was not issued by October 31, 2020…and Pfizer expects it will be unable to timely complete performance, then the Parties will discuss in good faith a contract modification to shift forward the estimated delivery schedule to reflect the difference in time period between October 31, 2020, and the date of actual regulatory approval or authorization.” In the SOW, Pfizer committed to notify the Government “of any event, risk, formal or informal FDA communication, or any other issue that would be reasonably expected to materially change the anticipated schedule by one week or more.” (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, pp. 13-14.)
On the date of Pfizer Protocol Amendment 9, October 29, 2020, Pfizer already had more than 62 evaluable efficacy cases diagnosed, as required in that exact amendment. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935)
On November 9, 2020, Pfizer announced that its BNT162b2 vaccine candidate was >90% effective based on a November 8, 2020, interim analysis of partial data from its Phase 3 clinical trial. (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/.)
Despite Pfizer not making its vaccine efficacy announcement until November 9, 2020 (https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3), more than one week after the October 31, 2020, SOW commitment date, there is no evidence that Pfizer notified the Government, as required, of a greater than one-week change in schedule. (Department of Defense (DOD)-Pfizer Contract W15QKN21C0012 (includes Modification 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.) (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/.)
On November 18, 2020, Pfizer reported 95% effectiveness based on analysis of a larger dataset that included 170 patients (162 in the placebo group and 8 in the vaccinated group). Based on the strength of that data, Pfizer formally requested Emergency Use Authorization (EUA) from the US Food and Drug Administration.” (Department of Defense (DOD)-Pfizer Contract W15QKN21C0012 (includes Modification 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.)
The FDA then authorized the EUA for Pfizer’s COVID-19 vaccine on December 11, 2020. (https://www.fda.gov/media/144416/download)
From the analysis of the 170 patients found in the FDA-released Pfizer clinical trial documents (https://phmpt.org/pfizer-16-plus-documents/), there is evidence that Pfizer had the data needed for its interim analysis of the “evaluable efficacy” in October 2020, when the various accrual thresholds of evaluable efficacy cases (i.e., the total number of COVID-19-positive patients that Pfizer counted to prove “efficacy”) were reached. Therefore, an announcement of vaccine efficacy could have been made in October 2020, before the U.S. 2020 presidential election. When you read on, you will likely have the same question we do: Was this unnecessarily delayed announcement an effort to negatively impact one candidate’s chances of winning the U.S. Presidential election?
On July 21, 20202, the U.S. government and Pfizer/BioNTech signed a Statement of Work (SOW) for COVID-19 vaccine development. (Technical Direction Letter for Medical CRBN Defense Consortium (MCDC), Request for Prototype Proposals (RPP) 20-11, Objective PRE-20-11 for “COVID-19 Pandemic — Large Scale Vaccine Manufacturing Demonstration’ (Pfizer, Inc.).” HHS.gov, 21 July 2020. Retrieved from https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf.) The goal of the prototype program was to “…rapidly develop and obtain regulatory licensure for a COVID-19 vaccine for use in adults ≥18 years of age.” According to the SOW, the goal was to have an FDA-approved or FDA-authorized vaccine ready for administration in the United States by October 31, 2020.
(Technical Direction Letter for Medical CRBN Defense Consortium (MCDC), Request for Prototype Proposals (RPP) 20-11, Objective PRE-20-11 for “COVID-19 Pandemic — Large Scale Vaccine Manufacturing Demonstration’ (Pfizer, Inc.).” HHS.gov, 21 July 2020. Retrieved from https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, p. 3.)
The scope of the prototype project (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, p. 10) was “…the demonstration by Pfizer of the supply and logistics capability to manufacture and distribute to the Government of 100M doses of a novel mRNA-based vaccine that has received FDA approval or authorization based on demonstration of efficacy (hereafter FDA-approved or authorized). “
Due to the perceived need for rapid vaccine development, the Pfizer/BioNTech trial for this vaccine, U.S. study C4591001, was designed as a single, multistage, and multiphase trial. As such, the trial protocol would be amended continuously whilst the clinical trial was underway. A trial was run as outlined in the protocol, and major modifications to the protocol are reflected in formal protocol amendments.
Phases 1 and 2 of the vaccine candidates (i.e., the possible vaccine formulations) had been ongoing since May 2020. With the rapidly evolving pandemic situation, there was a perceived need to demonstrate vaccine efficacy as soon as possible. Protocol Amendment 4 (June 30, 2020) had the first interim analysis plan. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 1792.) There were to be four interim analyses performed by an unblinded statistical team after accrual of 32, 62, 92, and 120 COVID-19 cases that met the trial’s primary endpoint (i.e., the “evaluable efficacy population,” which will be explained in detail). The goal was a total of 164 “evaluable efficacy” cases. This is mentioned on page 7 of the SOW, stating that the “…study currently is being amended to incorporate a pivotal efficacy study design.”
Protocol Amendment 5, dated July 24, 2020, clarified that a single vaccine candidate, BNT162b2 at a dose of 30 μg administered as two doses 21 days apart, would be studied in Phase 2/3 of the trial. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,. p. 1539) The official start date for Phase 3 was July 27, 2020, six days after the SOW was signed. From pages 13 and 14 of the SOW, “…if FDA approval or authorization was not issued by October 31, 2020…and Pfizer expects it will be unable to timely complete performance, then the Parties will discuss in good faith a contract modification to shift forward the estimated delivery schedule to reflect the difference in time period between October 31, 2020 and the date of actual regulatory approval or authorization.” (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf) Also, in addition to regular reporting requirements, during the period of performance, Pfizer committed to notify the government of any event, risk, formal or informal FDA communication, or any other issue that would be reasonably expected to materially change the anticipated schedule by ONE WEEK or more. (pp. 13-14, bold and capitalization added.)
No COVID-19 vaccine was approved by October 2020. A contract modification simply stated, “On November 9, 2020, Pfizer announced that BNT162b2 was >90% effective based on interim analysis of partial data from its Phase 3 clinical trial. On November 18, 2020, Pfizer reported 95% effectiveness based on analysis of a larger dataset that included 170 volunteers (162 in the placebo group and 8 in the vaccinated group). Based on the strength of this data, Pfizer formally requested Emergency Use Authorization (EUA) from the US Food and Drug Administration.” (Department of Defense (DOD)-Pfizer Contract W15QKN21C0012 (includes Modification 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.)
(DoD-Pfizer Contract W15QKN21C0012 (includes Mod 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.)
Previous reporting shows that the 170 patients formed the basis of the EUA application and approval. (Kunadhasan, J., Clark, E., & Flowers, C. (October 21, 2022). “Report 42: Pfizer’s EUA Granted Based on Fewer Than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant EUA.” Retrieved from https://dailyclout.io/report-41-the-170-clinical-trial-participants-who-changed-the-world-pfizer-ignored-protocol-deviations-to-obtain-emergency-use-authorization-for-its-covid-19-mrna-vaccine/.)
What is the evaluable efficacy population?
The evaluable efficacy population is the primary endpoint of Pfizer’s trial. An endpoint is a measurable outcome used to determine whether a drug under investigation is beneficial or not. To qualify to be part of the evaluable efficacy population, all eligible, randomized participants must:
Receive all vaccination(s) as randomized within the predefined window.
Have no evidence of COVID infection prior to seven days after the second dose of the vaccine.
Have the efficacy measurement (i.e., the test confirming symptomatic COVID-19 infection) only after seven days following the second vaccine dose.
Have no other major protocol deviations as determined by the clinician.
A major protocol deviation excluded a participant from the evaluable efficacy population from the date that it occurred through the participant’s remaining follow-up. Vaccine efficacy is measured by calculating the risk of disease among the vaccinated and placebo groups and determining the percentage reduction in disease between the two groups.
These COVID cases among these 170 patients were the basis of the claim of “95% effective.” We had already published that, in the 170 patients, five had dosing interval irregularities, one did not receive the correct dose of the investigational product, and another received a blood product within 60 days (a confounding event for infection), all of which should have disqualified them from being part of the evaluable efficacy population. Two others had been withdrawn from the trial prior to issuance of the Emergency Use Authorization (EUA). These disqualified patients would have brought the final number of cases to fewer than the 164 patient threshold, thus bringing into question if an EUA application could have been made, much less approved. To date there is no list available as to what constitutes a “major” protocol deviation that clinicians can review to discern if certain protocol deviations, presumably now reincluded into the efficacy analysis, have a significant effect on treatment efficacy.
When were the 170 evaluable efficacy cases diagnosed?
In our analysis, we have made no attempt to critique the study design and endpoints chosen. We are only assessing if the protocol was followed and if public statements by Pfizer correlate with what we found in the FDA-released Pfizer documents [https://phmpt.org/pfizer-16-plus-documents/].
In this report, we will focus on the timing of the accrual of the 170 evaluable efficacy patients.
First, one must understand what the public was led to believe.
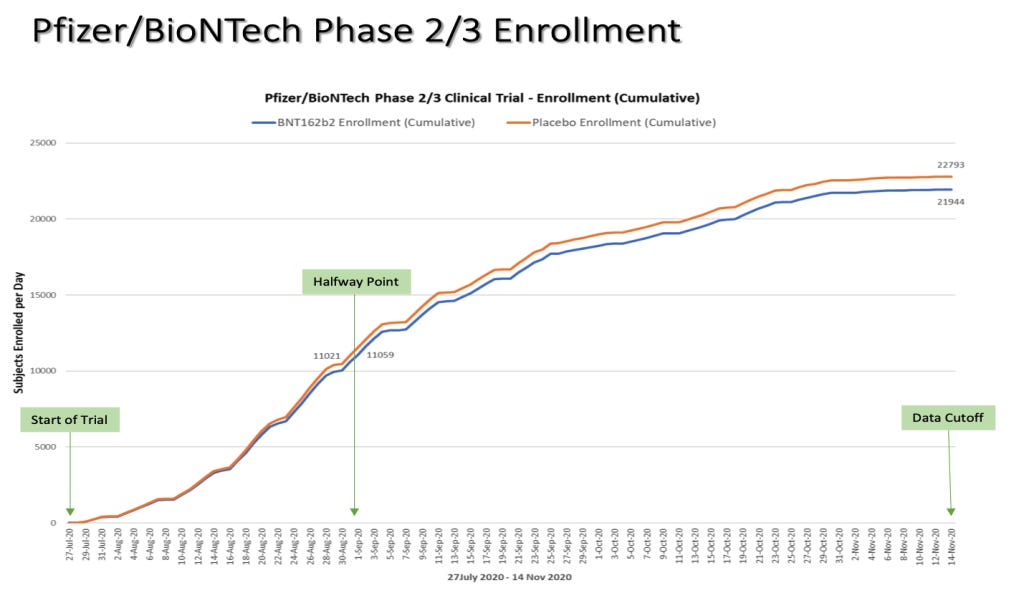
The Phase 3 portion of the trial started on July 27, 2020. Half of the Phase 3 trial participants were recruited by September 1, 2020.
Chart by Ed Clark
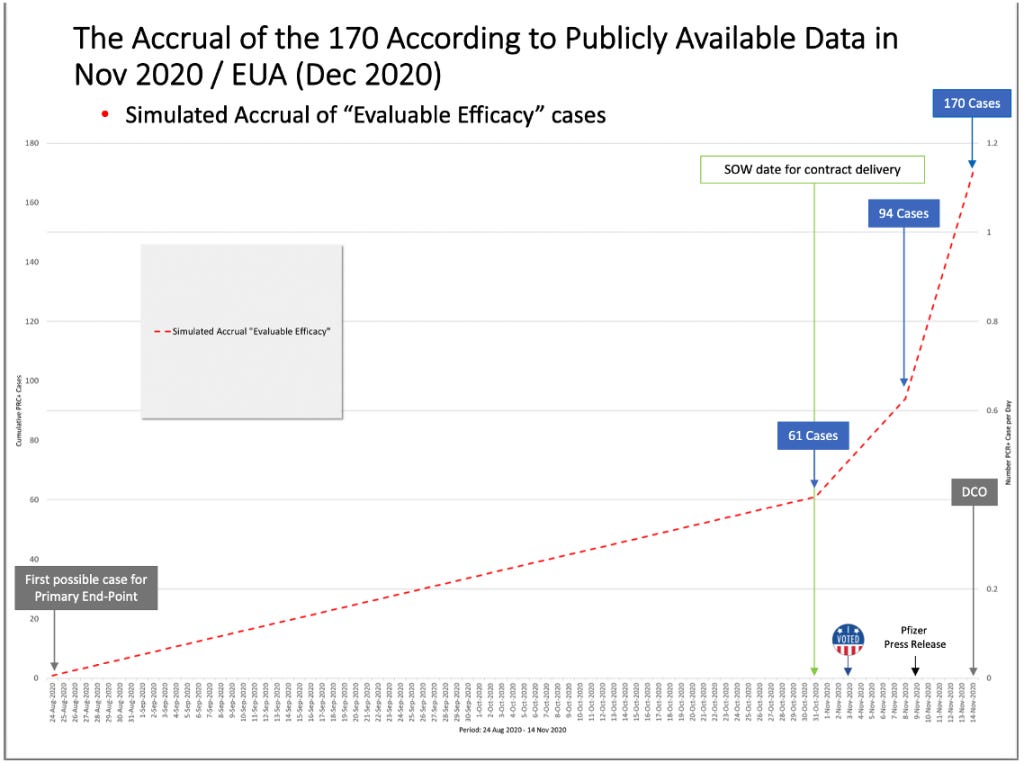
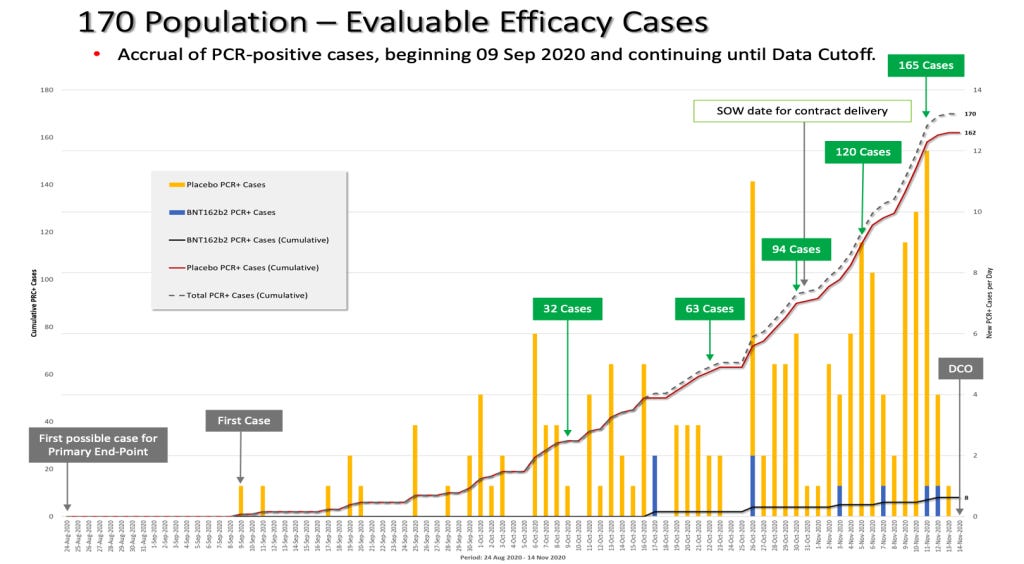
On October 29, 2020, a formal protocol amendment (the ninth) was made and clarified that interim analyses would be conducted after accrual of at least 62, 92, and 120 cases. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935) Given that there was an October 29 protocol amendment made stating that an interim analysis would be “…conducted after accrual of at least 62 cases…,” fewer than 62 cases must have diagnosed by that date.
(125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. Retrieved from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935.)
What the public was led to believe without access to FDA-released Pfizer documents and data
DCO = Data Cut-Off
Based on the October 29, 2020, protocol amendment stating that an interim analysis would be done when there were at least 62 cases accrued, and the lack of vaccine approval by October 31, 2020, fewer than the 62 cases needed for Interim Analysis 2 must have accumulated by the end of October 2020. It is unknown how many “evaluable efficacy” cases were actually confirmed by Pfizer by that date which enabled the DoD and Pfizer/BioNTech contract to be modified in “good faith.”
Yet, by the month of October 2020, more than half of the trial participants had already been recruited and completed the two-dose vaccination schedule, thus reaching the eligible timeframe to be included in the primary endpoint evaluable efficacy population. With a two-dose interval of 21 days and seven days post Dose 2, the first possible date to be eligible for the primary endpoint was August 24, 2020; however, the apparent rate of accrual of eligible cases from the August 24, 2020, to October 31, 2020, a period of 67 days, was only about 0.9 cases per day, or 61 cases over 67 days. (Note that the accrual rate must be derived from available documents and Pfizer’s statements since Pfizer and the DoD did not announce the number of evaluable efficacy cases through the end of October.)
After evaluating 94 confirmed cases of COVID-19 in trial participants in its first interim analysis conducted on November 8, 2020, Pfizer announced in a press release on November 9, 2020, that the company’s vaccine candidate was found to be more than 90% effective at preventing COVID-19. (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/.)
Hence, from October 31, 2020, until November 8, 2020, there was a jump in cases diagnosed from 61 to 94, a rate of about four cases a day. Then, an even bigger jump of cases occurred from November 8, 2020, through November 14, 2020 (the data cutoff date), from 94 cases to the final 170 cases or about 12 cases diagnosed per day.
In analyzing this, we also noted the inconsistencies in Pfizer’s public statements and the dates noted in official documentation. For example, on page 17 of the EUA documentation authorizing the vaccine it stated, “The date for data cut-off for the first interim analysis for efficacy was November 4, 2020, when a total of 94 confirmed COVID-19 cases were accrued.” (Gruber, M. F. (December 11, 2020). EUA Decision Memorandum. Retrieved from https://www.fda.gov/media/144416/download.) This is inconsistent with an article in USA Today that stated, “Pfizer lacked access to its trial data until after Election Day and could not have known or released the results prior to that. Albert Bourla, Pfizer’s CEO, told Axios that the data came in on Nov. 5 or 6, after Election Day on Nov. 3.” (Caldera, C. (2020, November 18). Fact check: Pfizer received COVID-19 vaccine data after Election Day, released within days. Retrieved June 19, 2023, from https://www.usatoday.com/story/news/factcheck/2020/11/18/fact-check-pfizer-received-covid-vaccine-data-after-election-day/6267242002/.) This begs the question as to how there could been the required “good faith” in fulfilling the contract by October 31, 2020, with a process that appears to have been set up to look into the data only after November 3, 2020, the date of the U.S. Presidential election.
When were the 170 evaluable efficacy cases diagnosed?
As we have found the 170 patients upon which the efficacy criterion for the EUA approval were based, we have also been able to plot the date of the accrual of the 170 patients. We were able to obtain the diagnosis date by combing through pages 1059 to 2506 of the Pfizer narrative documents released by the FDA. (125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf. (2022, July 1). Retrieved from https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf.) One caveat is that, at times, the diagnosis date was not explicitly stated in the documents, in which case the swab date was used as a proxy date. The narrative document was finalized on December 4, 2020. In another document, finalized on April 1, 2021, one is able to use the list of 170 patients to get the dates that the central lab swab was taken by going through pages 226 to 416. (125742_S1_M5_5351_c4591001 interim mth6 lab measurements sensitive.pdf. (2022, July 1). Retrieved from https://www.phmpt.org/wp-content/uploads/2022/07/125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf, pp. 1059-2506.) In another set of documents dated June 2, 2021, one can find the dates when the 170 patients’ central swab tests returned a positive result. The swabs were presumably processed only in Pfizer’s lab in Pearl River, New York, since it is the company’s “primary location for…global Vaccine Research and Development.” (https://www.pfizer.com/science/centers) (125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). Retrieved from https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
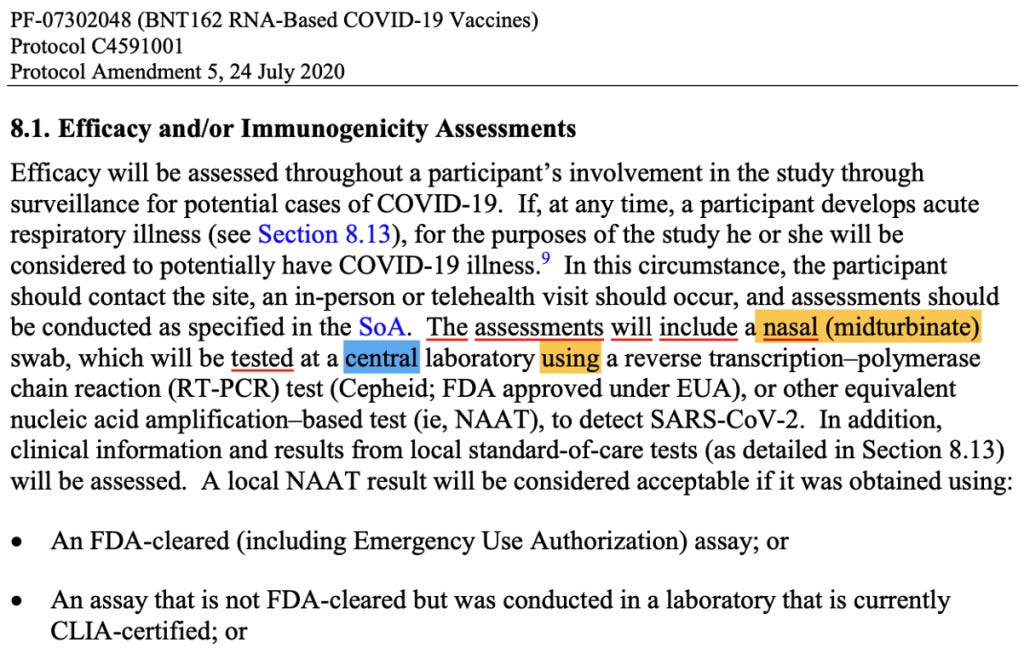
Publicly available Pfizer documentation causes confusion as to when precisely patients were diagnosed with COVID-19. If we look at Pfizer’s protocol, the diagnosis of cases was supposed to happen based on the results of a central laboratory test. There are certain conditions when the test results could be based on a local test.
(125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. (n.d.). Retrieved from https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf page 1591.)
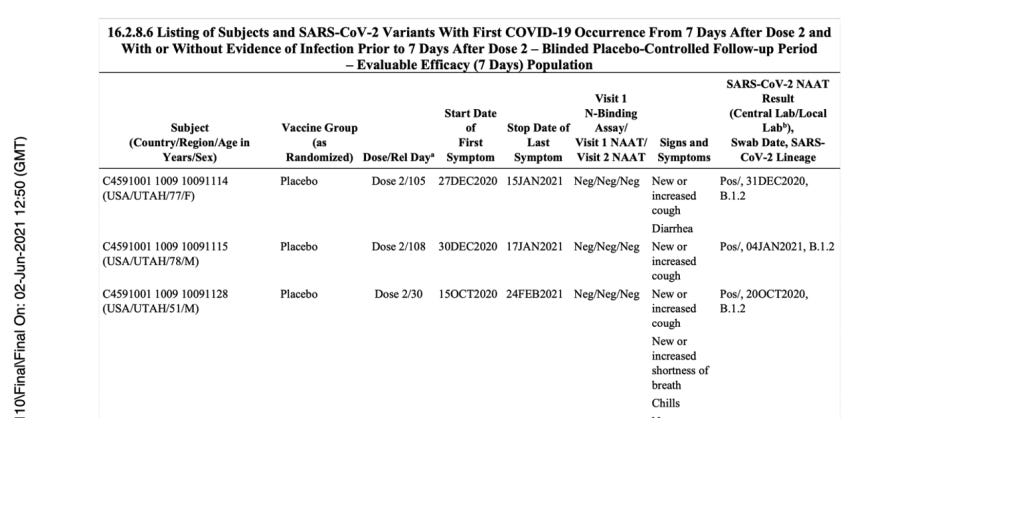
Let us review the example of a patient, ID 10091128.
(125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf. (2022, July 1). Retrieved from https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf, p. 1163.)
As you can see, the date of diagnosis is explicitly stated as October 16, 2020, even though the central lab test was done on October 19, 2020, as shown in the document finalized on December 4, 2020 (presumably a document used to apply for EUA, which was approved just one week later on December 11, 2020). (https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf) However, for that same patient, in a third document finalized in June 2021, the date of the central lab test is October 20, 2020. If taking that as a date of diagnosis for this patient, it would now be four days apart from the previous date of October 16, 2020.
(125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). Retrieved from https://phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf, p. 16.)
In December 2022, we published this approximate accrual of when the 170 subjects were diagnosed based on the interim narrative documents, explicitly stating date of diagnosis in the Pfizer documents finalized on December 4, 2020. (Kunadhasan, Jeyanthi. (2022, December 8). “170 patients that changed everything.” spectator.com.au. Retrieved June 19, 2023, from
https://www.spectator.com.au
. Kunadhasan, Jeyanthi. “‘170 Patients That Changed Everything’ – Spectator: Australia.” DailyClout, 8 Dec. 2022, https://dailyclout.io/170-patients-that-changed-everything-spectator-australia/.)
(Note the disproportionate excluded vaccinated patients versus placebo patients in the run up to the data cut-off date. This requires separate analysis and will not be covered in this report.)
We have analyzed the differing accruals of the 170 population based on documents finalized in December 2020 (https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf) and June 2021 (https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf) and further cross-checked with data from the FDA-released Pfizer XPT database files.
We have also superimposed the rate of accrual of the diagnosis of the 170 population comparing the two differing documents which were finalized at different times about six to seven months apart.
As one can see, the threshold number of efficacy cases is reached around the same time regardless.
Was Pfizer publicly forthright about the time required to accrue the 170 patients?
Now, let us superimpose the two graphs of accrual, the derived one (in red) based on Pfizer’s published documentation, which we introduced earlier, versus the actual accrual (in black) that has been based on the Pfizer document finalized in June 2021 (https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf).
Slide by Ed Clark and Jeyanthi Kunadhasan
Comparing the derived rate of accrual with the actual accrual, we can see a curious discrepancy in the number of evaluable cases diagnosed. Pfizer had accumulated at least 94 cases by October 31, 2020, but it delayed this announcement until a week later on November 9, 2020. What caused this delayed announcement? As highlighted earlier in the SOW (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, p. 14), the U.S. government would have had to have been informed of any issue that “…would be reasonably expected to materially change the anticipated schedule by one week or more.” October 31 to November 9 equates to 10 total days, more than the one week stated in the SOW, yet there is no evidence that the U.S. government was informed of this schedule change.
The rate of accumulation of the actual 170 cases in no way resembles what the public was led to believe by Pfizer and legacy media. In October 2020, the rate of accrual of cases is approximately 2.8 cases per day, which is not surprising given that more than half of the trial population had already been recruited by then, thus increasing one’s likelihood of success given the larger pool of participants. By November 8, 2020, there were about 134 COVID cases diagnosed. Why did Pfizer only admit to 94 diagnosed cases as of that date? Even accounting for a few days to evaluate cases, why is there a discrepancy of 40 cases?
The timing of the interim analyses
Remember that the SOW aspired for an FDA-approved or FDA-authorized vaccine by October 31, 2020. In his book Moonshot, Albert Bourla talks about his intention to have a vaccine by October 2020. (Bourla, Albert. (2022). Moonshot. Harper Business, p. 42.) Let us familiarize ourselves with the amendment talked about in the SOW as well as Protocol Amendment 4, the plan for interim analysis of evaluable cases.
(125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. (n.d.). Retrieved from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf p. 1793.)
Realistically, a vaccine could have only been approved by October 31, 2020, if the first interim analysis had been done at the accrual of 32 cases. This goalpost changed when individuals from the FDA’s Office of Vaccines Research and Review (OVRR), Center for Biologics Evaluation and Research (CBER) penned an article in the New England Journal of Medicine on October 16, 2020, outlining a new requirement that data from Phase 3 studies to support an EUA, which may result from a protocol-specified interim analysis, include a median follow-up duration of at least two months of safety data after completion of the full vaccination regimen. (Krause, P. R., & Gruber, M. F. (2020). Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. The New England Journal of Medicine, 383(19), e107. https://doi.org/10.1056/nejmp2031373.)
The newly imposed safety criteria could only have been fulfilled in the third week of November 2020, as was outlined in Pfizer’s press release (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” (n.d.). Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/) and the EUA documentation (Gruber, M. F. (December 11, 2020). EUA Decision Memorandum. Retrieved from https://www.fda.gov/media/144416/download,) section 4.2.2, p. 17). This is because half of the trial population had been recruited only on September 1, 2020. Pfizer only could have completed the stated vaccination regime for those recruited on September 1, 2020, 21 days later on September 22, 2020. Two months after September 22 is the third week of November, not November 9, 2020, when the interim analysis results were announced. Pfizer was allowed to circumvent the new requirement for a median follow-up of at least two months of safety data after completion of the full vaccination regimen. If the requirement was not going to be followed, why did Pfizer not announce its efficacy findings when 62 cases were accrued, immediately after October 22, 2020, as was promised in the ninth protocol amendment?
We have analyzed when the interim analyses could have been done, as well as estimated vaccine efficacy, according to the dates when enough cases were diagnosed from the 170-patient population.
Accrual of the 170-patient population Pfizer documents in intervals where the interim analyses could have been done. (125742_S6_M5_5351_C4591001 Sequencing listings.pdf, finalized June 2, 2021, https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
Slide by Ed Clark and J. Kunadhasan
Days’ difference between onset of symptoms and a positive central lab test
In doing this analysis, we have depended on the published Pfizer PDF documents that were released under court order by the FDA. We also have access to the Pfizer XPT database files released by the FDA, which are closer to the raw data Pfizer employees or contractors originally entered. As such, we can double-check the dates published in PDFs for dates the tests were taken and results returned, as well as when symptoms were first reported by patients. The ability to analyze the dates of the positive NAAT (nasal swab) test results from the XPT database was not possible until the January 3, 2023, release of Pfizer data by the FDA, which provided the subject-level .XPT files that contained those data. The data were included in the FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.XPT file (SAS export file for Signs and Symptoms of Disease Analysis Dataset, https://phmpt.org/wp-content/uploads/2023/01/FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.zip). This file was uploaded to the Abstractor website (https://vaccines.shinyapps.io/abstractor/) shortly after it was available and, thus, allowed access to researchers. Additional cross-checks were done with another file delivered in February 2023: FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.XPT (COVID-19 Efficacy Analysis Dataset, https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/020123/FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.zip).
The endpoint in this trial was having a symptomatic COVID infection. This was classified as an “illness visit” in the protocol with patients optimally coming in or having a telehealth consultation within three days of potential illness. (125742_S1_M5_5351_c4591001 interim mth6 lab measurements sensitive.pdf. (2022, July 1). Retrieved from https://www.phmpt.org/wp-content/uploads/2022/07/125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf, p. 1629.)
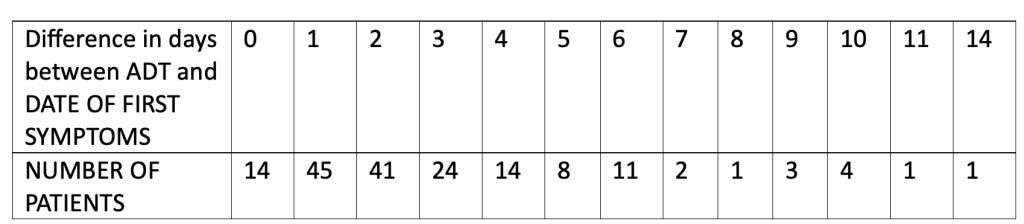
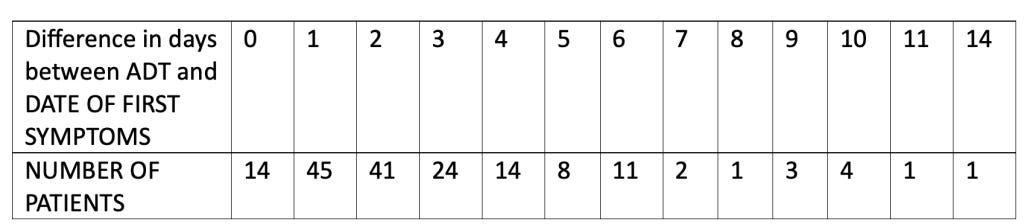
We analyzed the number of days’ difference between the patient reporting symptoms and the central lab test of the nasal swab returning a positive test result. This table shows the number of days’ difference between the Analysis Date for Test (ADT) – i.e., the date the central lab test result was recorded – minus the start date of first symptoms as found in the XPT database files.
Note that the total equals 169 patients since patient 10911203 did not have a central lab test performed.
ADT (Analysis Date for Test) for RTCOV2NS, CEPHEID RT-PCR ASSAY FOR SARS-COV-2
Most patients, 124 (72.9%), had a confirmatory central lab PCR test for COVID-19 between zero and three days of onset of symptoms, as was optimally suggested for in the protocol. Fourteen patients were able to have a confirmatory nasal swab central lab test, presumably done in the Pearl River facility in New York, on the very day their symptoms started.
So, for 14 people, scattered around the U.S. as well as Argentina, the number of days between the start of symptoms and a central lab test, most likely done in New York state, record of the related nasal swab was zero days. Nine of the 14 patients reported start of symptoms between November 5, 2020, and November 12, 2020. The patient from Argentina (subject ID 12311560) started having COVID symptoms on November 12, 2020, and the confirmatory positive nasal swab was able to be processed in New York that same day. Note that the flight from Buenos Aires to New York City metro area airports is approximately 11 hours. Then, add to that the time needed clear customs with a contagious biological specimen plus the approximate hour courier drive to Pfizer’s lab in Pearl River. This test seems to have, indeed, been completed at Pfizer’s “speed of science” (
). Please note, we do not have access to data such as when the time sample was received by a lab, the time the sample was processed, or when it was in the hands of the courier. We have access to when the data was recorded positive by the presumed central laboratory.
For those who had four or more days, up to a total of 14 days, between the onset of symptoms and a confirmatory central lab positive test of infection, 34 of the 45 (75.6%) had onset of symptoms between October 1, 2020, and November 3, 2020. Is there utility in attempting to diagnose a viral respiratory illness up to 14 days after the onset of symptoms? (Pretorius, M., & Venter, M. (2017). Diagnosis of Viral Infections. Viral Infections in Children, Volume I, 151–182. https://doi.org/10.1007/978-3-319-54033-7_6)
We would also like to highlight one of the patients, Subject 11231256, who in the .XPT files is listed as having symptoms from the October 24, 2020. However, in the PDFs and interim narrative files has the start of symptoms listed for Subject 11231256 is October 28, 2020. This patient has a nine-day difference between start of symptoms and having a positive test showing COVID-19 infection returned in the central lab. It would have shown up as only five days, a difference of four days, if taking data from the published PDF files without access to the XPT data. Which date is the correct date? Why does Pfizer have different dates in different documents for the same event and the same Subject?
Looking at this data as well, the accrual of the cases could have happened much earlier if patients had been able to have a central confirmatory test of infection closer to their onset of symptoms, as suggested in the protocol. Had swab results been processed in that way, more test results would have been available prior to the 2020 U.S. presidential election rather than after it.
In the study protocol, it was stated that for “…operational reasons, the interim analysis planned at accrual of 32 cases was removed.” (125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. Retrieved from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935.) It has never been explained clearly what exactly these “operational reasons” were. This change was formalized only on October 29, 2020, 13 days after the article about the new requirement of a median follow-up duration of at least two months of safety data after completion of the full vaccination regimen appeared in the NEJM on October 16, 2020. (Krause, P. R., & Gruber, M. F. (2020). Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. The New England Journal of Medicine, 383(19), e107. https://doi.org/10.1056/nejmp2031373.)
There was a discussion between a prominent cardiologist at the Scripps Research Institute, Eric Topol, and the then FDA Commissioner, Steven Hahn, on October 9, 2020, wherein Steven Hahn assured that any approval for the vaccine would be based on “science, not political convenience.” (“FDA’s Hahn to Topol: Decisions Will Be Made Based on Science Alone.” (October 9, 2020). Retrieved from https://www.medscape.com/viewarticle/938705.) (Kunadhasan, Jeyanthi. (March 24, 2023). the powerful politics of Covid vaccines. Spectator.com.au. Retrieved from
https://www.spectator.com.au
) Was there any peeking into the data then that precipitated the conversation? This is especially important as, officially, the first interim analysis was only admitted to having been completed on November 8, 2020. (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against) Have any questions been asked as to why Dr. Topol and Commissioner Hahn chose the seemingly fortuitous October 9 date to have such a discussion?
Eric Topol, the author of an open letter [Topol, E., MD. (August 31, 2020). “Dear Commissioner Hahn: Tell the Truth or Resign An Open Letter to Stephen Hahn, MD, From Eric Topol, MD.” Retrieved from https://www.medscape.com/viewarticle/936611] to Stephen Hahn on August 31, 2020, stated in his letter [https://www.medscape.com/viewarticle/936611#vp_3] stated, “…you will not, under any condition, authorize a SARS-CoV-2 vaccine approval before the full Phase 3 completion and read-out of a program.” He then posted this on Twitter two days after the discussion with Commissioner Stephen Hahn.
https://twitter.com/erictopol/status/1314979190555340800?s=61&t=N-rShViQ_hadKjOKEU-pRQ
If analyzed at accrual of 32 cases on October 9, 2020, the vaccine would have been 100% efficacious, as there had been no vaccinated patients diagnosed yet with COVID-19. If COVID-19 was such an emergency and this medication allegedly so lifesaving, what was the ethical justification for keeping this news from the public for another month? As we have already elucidated, the “…new safety requirement of median 2 month follow up for safety data…“ was not yet fulfilled when the announcement of the first interim analysis was made on November 9, 2020. (Krause, P. R., & Gruber, M. F. (2020). Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. The New England Journal of Medicine, 383(19), e107. https://doi.org/10.1056/nejmp2031373) If 32 cases were removed due to “operational reasons,” why not do the next interim analysis at accrual of more than 62 cases, which would have been immediately after October 22, 2020? This is extremely important, as the terms of the SOW contract could have been fulfilled if the first interim analysis had been done around October 9, 2020, when 32 cases were accrued.
In summary,
Slide by Ed Clark and Jeyanthi Kunadhasan
The green box highlights when 94 cases were actually accrued versus the blue box, when the official announcement was made by Pfizer after blowing past the contract delivery date of October 31, 2020.
As we can see, on the date of Protocol Amendment 9, October 29, 2020, there were already more than 62 evaluable efficacy cases diagnosed as required in that exact amendment. We have already highlighted previously that the dosing interval between Dose 1 and Dose 2 in this trial was changed WITHOUT a formal protocol amendment, so this makes us take more seriously any protocol amendment that has been made. (Kunadhasan, J, Clark, E., & Flowers, C. (October 21, 2022). “Report 42: Pfizer’s EUA Granted Based on Fewer Than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant EUA.” Retrieved from https://dailyclout.io/report-41-the-170-clinical-trial-participants-who-changed-the-world-pfizer-ignored-protocol-deviations-to-obtain-emergency-use-authorization-for-its-covid-19-mrna-vaccine/)
Is it reasonable to ask if the number of evaluable cases diagnosed – i.e., the pivotal efficacy data – was a deliverable closely monitored by all parties to the SOW? After all, the SOW mentioned this “pivotal efficacy portion.” (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, pp. 6-7.) For the contract to have been extended in good faith, what was the number of evaluable efficacy cases admitted to by Pfizer when the contract had to be modified after October 31, 2020? The late modification of the trial protocol on October 29, 2020, stating that the interim analysis would be done when there were at least 62 cases diagnosed, leads us to the obvious question — was the number of evaluable efficacy cases fraudulently underrepresented as to when they been diagnosed?
Very few people will know the answer to that question, and much of the SOW and the subsequent contract modification remain redacted. (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf) So, we are unable to answer this simple but vitally important question. We hope by raising awareness regarding this issue, so the public can get answers.
Data Methods for Determining the Timing of the 170 Patients’ Data
The ability to analyze the dates of the positive NAAT test results was not possible until the January 3, 2023, data production which provided the subject-level .XPT data files that contained this data. The data were included in the FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.XPT (SAS export file for Signs and Symptoms of Disease Analysis Dataset). (https://phmpt.org/wp-content/uploads/2023/01/FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.zip) This file was uploaded to the Abstractor website (https://vaccines.shinyapps.io/abstractor/) shortly after it was available, which allowed access to researchers.
The fields of interest in this data set are:
USUBJID – Unique Subject Identifier
PARAMCD – Parameter Code
RTCOV2NS – CEPHEID RT-PCR ASSAY FOR SARS-COV-2 (This is the test that was run in Pearl River, New York)
SARSCOV2 – SEVERE ACUTE RESP SYNDROME CORONAVIRUS 2 (This is the local test.)
PARCAT1 – Parameter Category 1
AVALC – Analysis Value (C)
This provides the results of the analysis.
POS (Shows that there was a positive test result for the test in question.)
ADT – Analysis Date for Test
Date entered for the test result
ASDT – Analysis Start Date
Date that first symptoms were entered
Steps:
Get the USUBJID for the 170 patients.
Select the data from the Signs and Symptoms of Disease Analysis Dataset for the 170 patients.
Verify the date of the first sign and symptom of disease (PARCAT1 = SIGNS AND SYMPTOMS OF DISEASE, AVALC=Y, ASDT is the resulting date for the first sign and symptom of disease). This should match the date in the PDF 16.2.8.6. (125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). Retrieved from https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
Verify that the date of the New York validation matches the “Swab Date” listed in 16.2.8.6 125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). (Retrieved from https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
. The New York validation date is determined from the Symptoms of Disease Analysis (PARAMCD=RTCOV2NS, PARCAT1=VIROLOGY, AVALC=POS), and ADT is the resulting date when the test result said that there is a POS test result in New York.
Additional cross-checks were done against another file produced by the FDA in February 2023 (FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.XPT, COVID-19 Efficacy Analysis Dataset, https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/020123/FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.zip).
Report 76: Pfizer Had Data to Announce Its COVID-19 Vaccine’s Alleged “Efficacy” in October 2020. Why Did Pfizer Delay the Announcement Until November 9, 2020, Six Days After the 2020 U.S. Presidential Election?
July 10, 2023 • by Jeyanthi Kunadhasan, MD, FANZCA, and Ed Clark, MSE – Team 3
Executive summary
On July 21, 2020, the United States (U.S.) government, Pfizer, and BioNTech signed a contract, known as a Statement of Work (SOW), for the rapid development of and obtaining regulatory licensure for a COVID-19 vaccine by October 31, 2020, a few days prior to the November 3, 2020, U.S. Presidential election. Regulatory licensure was to be based on approval by the U.S. Food and Drug Administration (FDA) or authorization based on demonstration of efficacy. (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf)
The July 2020 SOW states, “…if FDA approval or authorization was not issued by October 31, 2020…and Pfizer expects it will be unable to timely complete performance, then the Parties will discuss in good faith a contract modification to shift forward the estimated delivery schedule to reflect the difference in time period between October 31, 2020, and the date of actual regulatory approval or authorization.” In the SOW, Pfizer committed to notify the Government “of any event, risk, formal or informal FDA communication, or any other issue that would be reasonably expected to materially change the anticipated schedule by one week or more.” (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, pp. 13-14.)
On the date of Pfizer Protocol Amendment 9, October 29, 2020, Pfizer already had more than 62 evaluable efficacy cases diagnosed, as required in that exact amendment. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935)
On November 9, 2020, Pfizer announced that its BNT162b2 vaccine candidate was >90% effective based on a November 8, 2020, interim analysis of partial data from its Phase 3 clinical trial. (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/.)
Despite Pfizer not making its vaccine efficacy announcement until November 9, 2020 (https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3), more than one week after the October 31, 2020, SOW commitment date, there is no evidence that Pfizer notified the Government, as required, of a greater than one-week change in schedule. (Department of Defense (DOD)-Pfizer Contract W15QKN21C0012 (includes Modification 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.) (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/.)
On November 18, 2020, Pfizer reported 95% effectiveness based on analysis of a larger dataset that included 170 patients (162 in the placebo group and 8 in the vaccinated group). Based on the strength of that data, Pfizer formally requested Emergency Use Authorization (EUA) from the US Food and Drug Administration.” (Department of Defense (DOD)-Pfizer Contract W15QKN21C0012 (includes Modification 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.)
The FDA then authorized the EUA for Pfizer’s COVID-19 vaccine on December 11, 2020. (https://www.fda.gov/media/144416/download)
From the analysis of the 170 patients found in the FDA-released Pfizer clinical trial documents (https://phmpt.org/pfizer-16-plus-documents/), there is evidence that Pfizer had the data needed for its interim analysis of the “evaluable efficacy” in October 2020, when the various accrual thresholds of evaluable efficacy cases (i.e., the total number of COVID-19-positive patients that Pfizer counted to prove “efficacy”) were reached. Therefore, an announcement of vaccine efficacy could have been made in October 2020, before the U.S. 2020 presidential election. When you read on, you will likely have the same question we do: Was this unnecessarily delayed announcement an effort to negatively impact one candidate’s chances of winning the U.S. Presidential election?
On July 21, 20202, the U.S. government and Pfizer/BioNTech signed a Statement of Work (SOW) for COVID-19 vaccine development. (Technical Direction Letter for Medical CRBN Defense Consortium (MCDC), Request for Prototype Proposals (RPP) 20-11, Objective PRE-20-11 for “COVID-19 Pandemic — Large Scale Vaccine Manufacturing Demonstration’ (Pfizer, Inc.).” HHS.gov, 21 July 2020. Retrieved from https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf.) The goal of the prototype program was to “…rapidly develop and obtain regulatory licensure for a COVID-19 vaccine for use in adults ≥18 years of age.” According to the SOW, the goal was to have an FDA-approved or FDA-authorized vaccine ready for administration in the United States by October 31, 2020.
(Technical Direction Letter for Medical CRBN Defense Consortium (MCDC), Request for Prototype Proposals (RPP) 20-11, Objective PRE-20-11 for “COVID-19 Pandemic — Large Scale Vaccine Manufacturing Demonstration’ (Pfizer, Inc.).” HHS.gov, 21 July 2020. Retrieved from https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, p. 3.)
The scope of the prototype project (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, p. 10) was “…the demonstration by Pfizer of the supply and logistics capability to manufacture and distribute to the Government of 100M doses of a novel mRNA-based vaccine that has received FDA approval or authorization based on demonstration of efficacy (hereafter FDA-approved or authorized). “
Due to the perceived need for rapid vaccine development, the Pfizer/BioNTech trial for this vaccine, U.S. study C4591001, was designed as a single, multistage, and multiphase trial. As such, the trial protocol would be amended continuously whilst the clinical trial was underway. A trial was run as outlined in the protocol, and major modifications to the protocol are reflected in formal protocol amendments.
Phases 1 and 2 of the vaccine candidates (i.e., the possible vaccine formulations) had been ongoing since May 2020. With the rapidly evolving pandemic situation, there was a perceived need to demonstrate vaccine efficacy as soon as possible. Protocol Amendment 4 (June 30, 2020) had the first interim analysis plan. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 1792.) There were to be four interim analyses performed by an unblinded statistical team after accrual of 32, 62, 92, and 120 COVID-19 cases that met the trial’s primary endpoint (i.e., the “evaluable efficacy population,” which will be explained in detail). The goal was a total of 164 “evaluable efficacy” cases. This is mentioned on page 7 of the SOW, stating that the “…study currently is being amended to incorporate a pivotal efficacy study design.”
Protocol Amendment 5, dated July 24, 2020, clarified that a single vaccine candidate, BNT162b2 at a dose of 30 μg administered as two doses 21 days apart, would be studied in Phase 2/3 of the trial. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,. p. 1539) The official start date for Phase 3 was July 27, 2020, six days after the SOW was signed. From pages 13 and 14 of the SOW, “…if FDA approval or authorization was not issued by October 31, 2020…and Pfizer expects it will be unable to timely complete performance, then the Parties will discuss in good faith a contract modification to shift forward the estimated delivery schedule to reflect the difference in time period between October 31, 2020 and the date of actual regulatory approval or authorization.” (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf) Also, in addition to regular reporting requirements, during the period of performance, Pfizer committed to notify the government of any event, risk, formal or informal FDA communication, or any other issue that would be reasonably expected to materially change the anticipated schedule by ONE WEEK or more. (pp. 13-14, bold and capitalization added.)
No COVID-19 vaccine was approved by October 2020. A contract modification simply stated, “On November 9, 2020, Pfizer announced that BNT162b2 was >90% effective based on interim analysis of partial data from its Phase 3 clinical trial. On November 18, 2020, Pfizer reported 95% effectiveness based on analysis of a larger dataset that included 170 volunteers (162 in the placebo group and 8 in the vaccinated group). Based on the strength of this data, Pfizer formally requested Emergency Use Authorization (EUA) from the US Food and Drug Administration.” (Department of Defense (DOD)-Pfizer Contract W15QKN21C0012 (includes Modification 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.)
(DoD-Pfizer Contract W15QKN21C0012 (includes Mod 310). December 22, 2020. (n.d.). Retrieved from https://www.keionline.org/misc-docs/FOIA/DoD-Pfizer-Contract-W15QKN21C0012-22Dec2020.pdf, p. 3.)
Previous reporting shows that the 170 patients formed the basis of the EUA application and approval. (Kunadhasan, J., Clark, E., & Flowers, C. (October 21, 2022). “Report 42: Pfizer’s EUA Granted Based on Fewer Than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant EUA.” Retrieved from https://dailyclout.io/report-41-the-170-clinical-trial-participants-who-changed-the-world-pfizer-ignored-protocol-deviations-to-obtain-emergency-use-authorization-for-its-covid-19-mrna-vaccine/.)
What is the evaluable efficacy population?
The evaluable efficacy population is the primary endpoint of Pfizer’s trial. An endpoint is a measurable outcome used to determine whether a drug under investigation is beneficial or not. To qualify to be part of the evaluable efficacy population, all eligible, randomized participants must:
Receive all vaccination(s) as randomized within the predefined window.
Have no evidence of COVID infection prior to seven days after the second dose of the vaccine.
Have the efficacy measurement (i.e., the test confirming symptomatic COVID-19 infection) only after seven days following the second vaccine dose.
Have no other major protocol deviations as determined by the clinician.
A major protocol deviation excluded a participant from the evaluable efficacy population from the date that it occurred through the participant’s remaining follow-up. Vaccine efficacy is measured by calculating the risk of disease among the vaccinated and placebo groups and determining the percentage reduction in disease between the two groups.
These COVID cases among these 170 patients were the basis of the claim of “95% effective.” We had already published that, in the 170 patients, five had dosing interval irregularities, one did not receive the correct dose of the investigational product, and another received a blood product within 60 days (a confounding event for infection), all of which should have disqualified them from being part of the evaluable efficacy population. Two others had been withdrawn from the trial prior to issuance of the Emergency Use Authorization (EUA). These disqualified patients would have brought the final number of cases to fewer than the 164 patient threshold, thus bringing into question if an EUA application could have been made, much less approved. To date there is no list available as to what constitutes a “major” protocol deviation that clinicians can review to discern if certain protocol deviations, presumably now reincluded into the efficacy analysis, have a significant effect on treatment efficacy.
When were the 170 evaluable efficacy cases diagnosed?
In our analysis, we have made no attempt to critique the study design and endpoints chosen. We are only assessing if the protocol was followed and if public statements by Pfizer correlate with what we found in the FDA-released Pfizer documents [https://phmpt.org/pfizer-16-plus-documents/].
In this report, we will focus on the timing of the accrual of the 170 evaluable efficacy patients.
First, one must understand what the public was led to believe.
The Phase 3 portion of the trial started on July 27, 2020. Half of the Phase 3 trial participants were recruited by September 1, 2020.
Chart by Ed Clark
On October 29, 2020, a formal protocol amendment (the ninth) was made and clarified that interim analyses would be conducted after accrual of at least 62, 92, and 120 cases. (“125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf,” https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935) Given that there was an October 29 protocol amendment made stating that an interim analysis would be “…conducted after accrual of at least 62 cases…,” fewer than 62 cases must have diagnosed by that date.
(125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. Retrieved from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935.)
What the public was led to believe without access to FDA-released Pfizer documents and data
DCO = Data Cut-Off
Based on the October 29, 2020, protocol amendment stating that an interim analysis would be done when there were at least 62 cases accrued, and the lack of vaccine approval by October 31, 2020, fewer than the 62 cases needed for Interim Analysis 2 must have accumulated by the end of October 2020. It is unknown how many “evaluable efficacy” cases were actually confirmed by Pfizer by that date which enabled the DoD and Pfizer/BioNTech contract to be modified in “good faith.”
Yet, by the month of October 2020, more than half of the trial participants had already been recruited and completed the two-dose vaccination schedule, thus reaching the eligible timeframe to be included in the primary endpoint evaluable efficacy population. With a two-dose interval of 21 days and seven days post Dose 2, the first possible date to be eligible for the primary endpoint was August 24, 2020; however, the apparent rate of accrual of eligible cases from the August 24, 2020, to October 31, 2020, a period of 67 days, was only about 0.9 cases per day, or 61 cases over 67 days. (Note that the accrual rate must be derived from available documents and Pfizer’s statements since Pfizer and the DoD did not announce the number of evaluable efficacy cases through the end of October.)
After evaluating 94 confirmed cases of COVID-19 in trial participants in its first interim analysis conducted on November 8, 2020, Pfizer announced in a press release on November 9, 2020, that the company’s vaccine candidate was found to be more than 90% effective at preventing COVID-19. (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/.)
Hence, from October 31, 2020, until November 8, 2020, there was a jump in cases diagnosed from 61 to 94, a rate of about four cases a day. Then, an even bigger jump of cases occurred from November 8, 2020, through November 14, 2020 (the data cutoff date), from 94 cases to the final 170 cases or about 12 cases diagnosed per day.
In analyzing this, we also noted the inconsistencies in Pfizer’s public statements and the dates noted in official documentation. For example, on page 17 of the EUA documentation authorizing the vaccine it stated, “The date for data cut-off for the first interim analysis for efficacy was November 4, 2020, when a total of 94 confirmed COVID-19 cases were accrued.” (Gruber, M. F. (December 11, 2020). EUA Decision Memorandum. Retrieved from https://www.fda.gov/media/144416/download.) This is inconsistent with an article in USA Today that stated, “Pfizer lacked access to its trial data until after Election Day and could not have known or released the results prior to that. Albert Bourla, Pfizer’s CEO, told Axios that the data came in on Nov. 5 or 6, after Election Day on Nov. 3.” (Caldera, C. (2020, November 18). Fact check: Pfizer received COVID-19 vaccine data after Election Day, released within days. Retrieved June 19, 2023, from https://www.usatoday.com/story/news/factcheck/2020/11/18/fact-check-pfizer-received-covid-vaccine-data-after-election-day/6267242002/.) This begs the question as to how there could been the required “good faith” in fulfilling the contract by October 31, 2020, with a process that appears to have been set up to look into the data only after November 3, 2020, the date of the U.S. Presidential election.
When were the 170 evaluable efficacy cases diagnosed?
As we have found the 170 patients upon which the efficacy criterion for the EUA approval were based, we have also been able to plot the date of the accrual of the 170 patients. We were able to obtain the diagnosis date by combing through pages 1059 to 2506 of the Pfizer narrative documents released by the FDA. (125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf. (2022, July 1). Retrieved from https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf.) One caveat is that, at times, the diagnosis date was not explicitly stated in the documents, in which case the swab date was used as a proxy date. The narrative document was finalized on December 4, 2020. In another document, finalized on April 1, 2021, one is able to use the list of 170 patients to get the dates that the central lab swab was taken by going through pages 226 to 416. (125742_S1_M5_5351_c4591001 interim mth6 lab measurements sensitive.pdf. (2022, July 1). Retrieved from https://www.phmpt.org/wp-content/uploads/2022/07/125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf, pp. 1059-2506.) In another set of documents dated June 2, 2021, one can find the dates when the 170 patients’ central swab tests returned a positive result. The swabs were presumably processed only in Pfizer’s lab in Pearl River, New York, since it is the company’s “primary location for…global Vaccine Research and Development.” (https://www.pfizer.com/science/centers) (125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). Retrieved from https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
Publicly available Pfizer documentation causes confusion as to when precisely patients were diagnosed with COVID-19. If we look at Pfizer’s protocol, the diagnosis of cases was supposed to happen based on the results of a central laboratory test. There are certain conditions when the test results could be based on a local test.
(125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. (n.d.). Retrieved from https://www.phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf page 1591.)
Let us review the example of a patient, ID 10091128.
(125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf. (2022, July 1). Retrieved from https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf, p. 1163.)
As you can see, the date of diagnosis is explicitly stated as October 16, 2020, even though the central lab test was done on October 19, 2020, as shown in the document finalized on December 4, 2020 (presumably a document used to apply for EUA, which was approved just one week later on December 11, 2020). (https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf) However, for that same patient, in a third document finalized in June 2021, the date of the central lab test is October 20, 2020. If taking that as a date of diagnosis for this patient, it would now be four days apart from the previous date of October 16, 2020.
(125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). Retrieved from https://phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf, p. 16.)
In December 2022, we published this approximate accrual of when the 170 subjects were diagnosed based on the interim narrative documents, explicitly stating date of diagnosis in the Pfizer documents finalized on December 4, 2020. (Kunadhasan, Jeyanthi. (2022, December 8). “170 patients that changed everything.” spectator.com.au. Retrieved June 19, 2023, from
https://www.spectator.com.au
. Kunadhasan, Jeyanthi. “‘170 Patients That Changed Everything’ – Spectator: Australia.” DailyClout, 8 Dec. 2022, https://dailyclout.io/170-patients-that-changed-everything-spectator-australia/.)
(Note the disproportionate excluded vaccinated patients versus placebo patients in the run up to the data cut-off date. This requires separate analysis and will not be covered in this report.)
We have analyzed the differing accruals of the 170 population based on documents finalized in December 2020 (https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf) and June 2021 (https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf) and further cross-checked with data from the FDA-released Pfizer XPT database files.
We have also superimposed the rate of accrual of the diagnosis of the 170 population comparing the two differing documents which were finalized at different times about six to seven months apart.
As one can see, the threshold number of efficacy cases is reached around the same time regardless.
Was Pfizer publicly forthright about the time required to accrue the 170 patients?
Now, let us superimpose the two graphs of accrual, the derived one (in red) based on Pfizer’s published documentation, which we introduced earlier, versus the actual accrual (in black) that has been based on the Pfizer document finalized in June 2021 (https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf).
Slide by Ed Clark and Jeyanthi Kunadhasan
Comparing the derived rate of accrual with the actual accrual, we can see a curious discrepancy in the number of evaluable cases diagnosed. Pfizer had accumulated at least 94 cases by October 31, 2020, but it delayed this announcement until a week later on November 9, 2020. What caused this delayed announcement? As highlighted earlier in the SOW (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, p. 14), the U.S. government would have had to have been informed of any issue that “…would be reasonably expected to materially change the anticipated schedule by one week or more.” October 31 to November 9 equates to 10 total days, more than the one week stated in the SOW, yet there is no evidence that the U.S. government was informed of this schedule change.
The rate of accumulation of the actual 170 cases in no way resembles what the public was led to believe by Pfizer and legacy media. In October 2020, the rate of accrual of cases is approximately 2.8 cases per day, which is not surprising given that more than half of the trial population had already been recruited by then, thus increasing one’s likelihood of success given the larger pool of participants. By November 8, 2020, there were about 134 COVID cases diagnosed. Why did Pfizer only admit to 94 diagnosed cases as of that date? Even accounting for a few days to evaluate cases, why is there a discrepancy of 40 cases?
The timing of the interim analyses
Remember that the SOW aspired for an FDA-approved or FDA-authorized vaccine by October 31, 2020. In his book Moonshot, Albert Bourla talks about his intention to have a vaccine by October 2020. (Bourla, Albert. (2022). Moonshot. Harper Business, p. 42.) Let us familiarize ourselves with the amendment talked about in the SOW as well as Protocol Amendment 4, the plan for interim analysis of evaluable cases.
(125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. (n.d.). Retrieved from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf p. 1793.)
Realistically, a vaccine could have only been approved by October 31, 2020, if the first interim analysis had been done at the accrual of 32 cases. This goalpost changed when individuals from the FDA’s Office of Vaccines Research and Review (OVRR), Center for Biologics Evaluation and Research (CBER) penned an article in the New England Journal of Medicine on October 16, 2020, outlining a new requirement that data from Phase 3 studies to support an EUA, which may result from a protocol-specified interim analysis, include a median follow-up duration of at least two months of safety data after completion of the full vaccination regimen. (Krause, P. R., & Gruber, M. F. (2020). Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. The New England Journal of Medicine, 383(19), e107. https://doi.org/10.1056/nejmp2031373.)
The newly imposed safety criteria could only have been fulfilled in the third week of November 2020, as was outlined in Pfizer’s press release (“Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study.” (n.d.). Retrieved June 19, 2023, from https://www.businesswire.com/news/home/20201109005539/en/) and the EUA documentation (Gruber, M. F. (December 11, 2020). EUA Decision Memorandum. Retrieved from https://www.fda.gov/media/144416/download,) section 4.2.2, p. 17). This is because half of the trial population had been recruited only on September 1, 2020. Pfizer only could have completed the stated vaccination regime for those recruited on September 1, 2020, 21 days later on September 22, 2020. Two months after September 22 is the third week of November, not November 9, 2020, when the interim analysis results were announced. Pfizer was allowed to circumvent the new requirement for a median follow-up of at least two months of safety data after completion of the full vaccination regimen. If the requirement was not going to be followed, why did Pfizer not announce its efficacy findings when 62 cases were accrued, immediately after October 22, 2020, as was promised in the ninth protocol amendment?
We have analyzed when the interim analyses could have been done, as well as estimated vaccine efficacy, according to the dates when enough cases were diagnosed from the 170-patient population.
Accrual of the 170-patient population Pfizer documents in intervals where the interim analyses could have been done. (125742_S6_M5_5351_C4591001 Sequencing listings.pdf, finalized June 2, 2021, https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
Slide by Ed Clark and J. Kunadhasan
Days’ difference between onset of symptoms and a positive central lab test
In doing this analysis, we have depended on the published Pfizer PDF documents that were released under court order by the FDA. We also have access to the Pfizer XPT database files released by the FDA, which are closer to the raw data Pfizer employees or contractors originally entered. As such, we can double-check the dates published in PDFs for dates the tests were taken and results returned, as well as when symptoms were first reported by patients. The ability to analyze the dates of the positive NAAT (nasal swab) test results from the XPT database was not possible until the January 3, 2023, release of Pfizer data by the FDA, which provided the subject-level .XPT files that contained those data. The data were included in the FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.XPT file (SAS export file for Signs and Symptoms of Disease Analysis Dataset, https://phmpt.org/wp-content/uploads/2023/01/FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.zip). This file was uploaded to the Abstractor website (https://vaccines.shinyapps.io/abstractor/) shortly after it was available and, thus, allowed access to researchers. Additional cross-checks were done with another file delivered in February 2023: FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.XPT (COVID-19 Efficacy Analysis Dataset, https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/020123/FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.zip).
The endpoint in this trial was having a symptomatic COVID infection. This was classified as an “illness visit” in the protocol with patients optimally coming in or having a telehealth consultation within three days of potential illness. (125742_S1_M5_5351_c4591001 interim mth6 lab measurements sensitive.pdf. (2022, July 1). Retrieved from https://www.phmpt.org/wp-content/uploads/2022/07/125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf, p. 1629.)
We analyzed the number of days’ difference between the patient reporting symptoms and the central lab test of the nasal swab returning a positive test result. This table shows the number of days’ difference between the Analysis Date for Test (ADT) – i.e., the date the central lab test result was recorded – minus the start date of first symptoms as found in the XPT database files.
Note that the total equals 169 patients since patient 10911203 did not have a central lab test performed.
ADT (Analysis Date for Test) for RTCOV2NS, CEPHEID RT-PCR ASSAY FOR SARS-COV-2
Most patients, 124 (72.9%), had a confirmatory central lab PCR test for COVID-19 between zero and three days of onset of symptoms, as was optimally suggested for in the protocol. Fourteen patients were able to have a confirmatory nasal swab central lab test, presumably done in the Pearl River facility in New York, on the very day their symptoms started.
So, for 14 people, scattered around the U.S. as well as Argentina, the number of days between the start of symptoms and a central lab test, most likely done in New York state, record of the related nasal swab was zero days. Nine of the 14 patients reported start of symptoms between November 5, 2020, and November 12, 2020. The patient from Argentina (subject ID 12311560) started having COVID symptoms on November 12, 2020, and the confirmatory positive nasal swab was able to be processed in New York that same day. Note that the flight from Buenos Aires to New York City metro area airports is approximately 11 hours. Then, add to that the time needed clear customs with a contagious biological specimen plus the approximate hour courier drive to Pfizer’s lab in Pearl River. This test seems to have, indeed, been completed at Pfizer’s “speed of science” (
). Please note, we do not have access to data such as when the time sample was received by a lab, the time the sample was processed, or when it was in the hands of the courier. We have access to when the data was recorded positive by the presumed central laboratory.
For those who had four or more days, up to a total of 14 days, between the onset of symptoms and a confirmatory central lab positive test of infection, 34 of the 45 (75.6%) had onset of symptoms between October 1, 2020, and November 3, 2020. Is there utility in attempting to diagnose a viral respiratory illness up to 14 days after the onset of symptoms? (Pretorius, M., & Venter, M. (2017). Diagnosis of Viral Infections. Viral Infections in Children, Volume I, 151–182. https://doi.org/10.1007/978-3-319-54033-7_6)
We would also like to highlight one of the patients, Subject 11231256, who in the .XPT files is listed as having symptoms from the October 24, 2020. However, in the PDFs and interim narrative files has the start of symptoms listed for Subject 11231256 is October 28, 2020. This patient has a nine-day difference between start of symptoms and having a positive test showing COVID-19 infection returned in the central lab. It would have shown up as only five days, a difference of four days, if taking data from the published PDF files without access to the XPT data. Which date is the correct date? Why does Pfizer have different dates in different documents for the same event and the same Subject?
Looking at this data as well, the accrual of the cases could have happened much earlier if patients had been able to have a central confirmatory test of infection closer to their onset of symptoms, as suggested in the protocol. Had swab results been processed in that way, more test results would have been available prior to the 2020 U.S. presidential election rather than after it.
In the study protocol, it was stated that for “…operational reasons, the interim analysis planned at accrual of 32 cases was removed.” (125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf. Retrieved from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-interim-mth6-protocol.pdf, p. 935.) It has never been explained clearly what exactly these “operational reasons” were. This change was formalized only on October 29, 2020, 13 days after the article about the new requirement of a median follow-up duration of at least two months of safety data after completion of the full vaccination regimen appeared in the NEJM on October 16, 2020. (Krause, P. R., & Gruber, M. F. (2020). Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. The New England Journal of Medicine, 383(19), e107. https://doi.org/10.1056/nejmp2031373.)
There was a discussion between a prominent cardiologist at the Scripps Research Institute, Eric Topol, and the then FDA Commissioner, Steven Hahn, on October 9, 2020, wherein Steven Hahn assured that any approval for the vaccine would be based on “science, not political convenience.” (“FDA’s Hahn to Topol: Decisions Will Be Made Based on Science Alone.” (October 9, 2020). Retrieved from https://www.medscape.com/viewarticle/938705.) (Kunadhasan, Jeyanthi. (March 24, 2023). the powerful politics of Covid vaccines. Spectator.com.au. Retrieved from
https://www.spectator.com.au
) Was there any peeking into the data then that precipitated the conversation? This is especially important as, officially, the first interim analysis was only admitted to having been completed on November 8, 2020. (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against) Have any questions been asked as to why Dr. Topol and Commissioner Hahn chose the seemingly fortuitous October 9 date to have such a discussion?
Eric Topol, the author of an open letter [Topol, E., MD. (August 31, 2020). “Dear Commissioner Hahn: Tell the Truth or Resign An Open Letter to Stephen Hahn, MD, From Eric Topol, MD.” Retrieved from https://www.medscape.com/viewarticle/936611] to Stephen Hahn on August 31, 2020, stated in his letter [https://www.medscape.com/viewarticle/936611#vp_3] stated, “…you will not, under any condition, authorize a SARS-CoV-2 vaccine approval before the full Phase 3 completion and read-out of a program.” He then posted this on Twitter two days after the discussion with Commissioner Stephen Hahn.
https://twitter.com/erictopol/status/1314979190555340800?s=61&t=N-rShViQ_hadKjOKEU-pRQ
If analyzed at accrual of 32 cases on October 9, 2020, the vaccine would have been 100% efficacious, as there had been no vaccinated patients diagnosed yet with COVID-19. If COVID-19 was such an emergency and this medication allegedly so lifesaving, what was the ethical justification for keeping this news from the public for another month? As we have already elucidated, the “…new safety requirement of median 2 month follow up for safety data…“ was not yet fulfilled when the announcement of the first interim analysis was made on November 9, 2020. (Krause, P. R., & Gruber, M. F. (2020). Emergency Use Authorization of Covid Vaccines — Safety and Efficacy Follow-up Considerations. The New England Journal of Medicine, 383(19), e107. https://doi.org/10.1056/nejmp2031373) If 32 cases were removed due to “operational reasons,” why not do the next interim analysis at accrual of more than 62 cases, which would have been immediately after October 22, 2020? This is extremely important, as the terms of the SOW contract could have been fulfilled if the first interim analysis had been done around October 9, 2020, when 32 cases were accrued.
In summary,
Slide by Ed Clark and Jeyanthi Kunadhasan
The green box highlights when 94 cases were actually accrued versus the blue box, when the official announcement was made by Pfizer after blowing past the contract delivery date of October 31, 2020.
As we can see, on the date of Protocol Amendment 9, October 29, 2020, there were already more than 62 evaluable efficacy cases diagnosed as required in that exact amendment. We have already highlighted previously that the dosing interval between Dose 1 and Dose 2 in this trial was changed WITHOUT a formal protocol amendment, so this makes us take more seriously any protocol amendment that has been made. (Kunadhasan, J, Clark, E., & Flowers, C. (October 21, 2022). “Report 42: Pfizer’s EUA Granted Based on Fewer Than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant EUA.” Retrieved from https://dailyclout.io/report-41-the-170-clinical-trial-participants-who-changed-the-world-pfizer-ignored-protocol-deviations-to-obtain-emergency-use-authorization-for-its-covid-19-mrna-vaccine/)
Is it reasonable to ask if the number of evaluable cases diagnosed – i.e., the pivotal efficacy data – was a deliverable closely monitored by all parties to the SOW? After all, the SOW mentioned this “pivotal efficacy portion.” (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf, pp. 6-7.) For the contract to have been extended in good faith, what was the number of evaluable efficacy cases admitted to by Pfizer when the contract had to be modified after October 31, 2020? The late modification of the trial protocol on October 29, 2020, stating that the interim analysis would be done when there were at least 62 cases diagnosed, leads us to the obvious question — was the number of evaluable efficacy cases fraudulently underrepresented as to when they been diagnosed?
Very few people will know the answer to that question, and much of the SOW and the subsequent contract modification remain redacted. (https://www.hhs.gov/sites/default/files/pfizer-inc-covid-19-vaccine-contract.pdf) So, we are unable to answer this simple but vitally important question. We hope by raising awareness regarding this issue, so the public can get answers.
Data Methods for Determining the Timing of the 170 Patients’ Data
The ability to analyze the dates of the positive NAAT test results was not possible until the January 3, 2023, data production which provided the subject-level .XPT data files that contained this data. The data were included in the FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.XPT (SAS export file for Signs and Symptoms of Disease Analysis Dataset). (https://phmpt.org/wp-content/uploads/2023/01/FDA-CBER-2021-5683-0663135-0671344-125742_S1_M5_c4591001-A-D-adsympt.zip) This file was uploaded to the Abstractor website (https://vaccines.shinyapps.io/abstractor/) shortly after it was available, which allowed access to researchers.
The fields of interest in this data set are:
USUBJID – Unique Subject Identifier
PARAMCD – Parameter Code
RTCOV2NS – CEPHEID RT-PCR ASSAY FOR SARS-COV-2 (This is the test that was run in Pearl River, New York)
SARSCOV2 – SEVERE ACUTE RESP SYNDROME CORONAVIRUS 2 (This is the local test.)
PARCAT1 – Parameter Category 1
AVALC – Analysis Value (C)
This provides the results of the analysis.
POS (Shows that there was a positive test result for the test in question.)
ADT – Analysis Date for Test
Date entered for the test result
ASDT – Analysis Start Date
Date that first symptoms were entered
Steps:
Get the USUBJID for the 170 patients.
Select the data from the Signs and Symptoms of Disease Analysis Dataset for the 170 patients.
Verify the date of the first sign and symptom of disease (PARCAT1 = SIGNS AND SYMPTOMS OF DISEASE, AVALC=Y, ASDT is the resulting date for the first sign and symptom of disease). This should match the date in the PDF 16.2.8.6. (125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). Retrieved from https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
Verify that the date of the New York validation matches the “Swab Date” listed in 16.2.8.6 125742_S6_M5_5351_c4591001 sequencing listings.pdf. (2023, January 3). (Retrieved from https://www.phmpt.org/wp-content/uploads/2023/01/125742_S6_M5_5351_c4591001-sequencing-listings.pdf.)
. The New York validation date is determined from the Symptoms of Disease Analysis (PARAMCD=RTCOV2NS, PARCAT1=VIROLOGY, AVALC=POS), and ADT is the resulting date when the test result said that there is a POS test result in New York.
Additional cross-checks were done against another file produced by the FDA in February 2023 (FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.XPT, COVID-19 Efficacy Analysis Dataset, https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/020123/FDA-CBER-2021-5683-0710069-0763793-125742_S1_M5_c4591001-A-D-adc19ef.zip).
170 Efficacy Population Analysis
(Download: 170 Efficacy Population Analysis in Excel)
Source: Mercola.com
Why Pfizer Stopped COVID Vax Trial
Analysis by Dr. Joseph Mercola
July 11, 2023
STORY AT-A-GLANCE
In February 2021, Pfizer launched a randomized, placebo-controlled, observer-blind study to evaluate the safety of its COVID-19 shot — BNT162b2 — in healthy pregnant women
The injections were set to take place between 24 and 34 weeks’ gestation, with participants randomized 1-to-1 to receive a COVID-19 shot or placebo
The study was initially set to enroll 4,000 women, but Pfizer only signed up 349, then stopped enrollment entirely
The CDC’s widespread endorsement of COVID-19 shots for pregnant women before the study was completed may have negated the need for the trial
Pfizer stated that because so many pregnant women had already gone ahead and gotten the shot, due to the government’s endorsement, enrollment dropped and there was no reason to move ahead with the study
No randomized trial data are available for use of the COVID-19 shot in pregnant women, and Pfizer cut its pregnancy trial short.1 But this doesn’t stop the U.S. Centers for Disease Control and Prevention from recommending COVID-19 injections for everyone 6 months and older, including “people who are pregnant, breastfeeding, trying to get pregnant now, or those who might become pregnant in the future.”2
“Not having any good data didn’t seem to bother the CDC,” Dr. Martin Makary, a professor at Johns Hopkins University School of Medicine, wrote in Tablet.3 He and a team of scientists petitioned the U.S. Food and Drug Administration to add a disclaimer to the shot’s label about the lack of trial data on its safety in pregnant women. The FDA declined, stating that this information wouldn’t be relevant.
Pregnant women, however, do need to know about the shot’s lack of testing during pregnancy — it’s the foundation of informed consent. They’d also likely be interested to know why Pfizer stopped its pregnancy trial early and has yet to make the results it did find public.
Pfizer Stopped COVID Jab Pregnancy Trial, Withholds Data
In February 2021, Pfizer launched a randomized, placebo-controlled, observer-blind study to evaluate the safety of its COVID-19 shot — BNT162b2 — in healthy pregnant women. The injections were set to take place between 24 and 34 weeks’ gestation, with participants randomized 1-to-1 to receive a COVID-19 shot or placebo.4 But while the study was initially set to enroll 4,000 women,5 Pfizer only signed up 349, then stopped enrollment entirely.6
“Most concerning,” Makary explained, “the pregnancy outcomes of those who participated in the trial, and their babies, are still not public today, nearly two years later.”7 Pregnant and breastfeeding women were excluded from Pfizer’s and Moderna’s phase III clinical trials,8 but in April 2021, CDC director Dr. Rochelle Walensky announced, "CDC recommends that pregnant people receive the COVID-19 vaccine."9
The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) soon echoed this statement, recommending pregnant women get injected, as did the UK’s Royal College of Obstetricians and Gynecologists.10
So, as the months passed, hundreds of thousands of pregnant women globally rolled up their sleeves to receive the vaccine, despite the absence of any clinical trials. “The message from authorities was clear — the harm from COVID-19 infection outweighed any harms from the mRNA vaccine — but in truth, they couldn’t possibly have known,” noted Maryanne Demasi, Ph.D., a former medical scientist with the University of Adelaide and former reporter for ABC News in Australia.11
Animal Studies Show Fetal Loss, Skeletal Variations
Pfizer’s product labeling suggested, “No vaccine-related adverse effects on female fertility, fetal development or postnatal development were reported.” But, Demasi reported, a freedom of information request from 2021 showed this was based on an animal study of just 44 rats, which showed concerning findings about fetal loss:12
“The study found the vaccine led to a statistically significant doubling in fetal loss (9.77% mRNA vs 4.09% saline), but Pfizer concluded that the difference between the two groups was “not biologically meaningful.”
The label also states that Pfizer’s mRNA vaccine was not tested for its potential to cause carcinogenicity (ability to cause cancer), genotoxicity (ability to damage genetic information), or impairment of male fertility.”
An animal study conducted by Moderna also showed a significant number of rats were born with skeletal variations after their mothers were injected with the COVID-19 shot while pregnant. But, according to Demasi, “Moderna concluded that the “Skeletal variations are structural changes that do not impact development or function of a developing embryo” and therefore “not considered adverse.””
Trial Stopped Because Many Pregnant Women Already Injected
Pfizer hasn’t publicly explained why its trial on pregnant women was stopped short, nor why the resulting data hasn’t been published. It’s possible they ended the trial because of unfavorable results. It wouldn’t be the first time.
However, in this case it appears the CDC’s widespread endorsement of COVID-19 shots for pregnant women — before the study was completed — may have negated the need for the trial altogether, at least from Pfizer’s perspective. As Makary put it:13
“In the case of the COVID vaccine trial in pregnant women, the trial may have been terminated not because the results were unfavorable, but because no data was needed. The medical and public health establishments had already made up their minds, declaring it safe and effective regardless of what the data was going to show. Why evaluate a product if the CDC and ACOG are already sold on the product?”
Demasi agreed. In fact, she published an email from Pfizer, in which the company admitted that enrollment for their trial significantly declined in the end of 2021, because health officials had already given the shot their seal of approval. Because so many pregnant women had already gone ahead and gotten the shot, due to the government’s endorsement, there was no reason to move ahead with the study. Demasi published Pfizer’s email response, which reads:14
“In the fourth quarter of 2021, enrollment was stopped in C4591015 Study (a Phase 2/3 placebo controlled randomized observer-blind study to evaluate the safety, tolerability, and immunogenicity of BNT162b2 against COVID-19 in healthy pregnant women 18 years of age and older). This study was developed prior to availability or recommendation for COVID-19 vaccination in pregnant women.
The environment changed during 2021 and by September 2021, COVID-19 vaccines were recommended by applicable recommending bodies (e.g., ACIP in the U.S.) for pregnant women in all participating/planned countries, and as a result the enrollment rate declined significantly.
With the declining enrollment, the study had insufficient sample size to assess the primary immunogenicity objective and continuation of this placebo controlled study could no longer be justified due to global recommendations. This proposal was shared with and agreed to by FDA and EMA.”
As for the lack of published data, Pfizer stated it doesn’t have it:15
“Pfizer does not yet have a complete data set from the maternal immunization study, C4591015. Pfizer and BioNTech plan to complete the analysis of the clinical trial C4591015 and share it with global public health regulators and seek publication or presentation as is our standard practice.
It is important to note that relevant real-world evidence on the use of COVID-19 vaccines in pregnant women has been presented and published numerous times by various parties in multiple journals and forums.”
Natural Immunity Is Still Being Ignored
Not only does ACOG “strongly recommend” that pregnant women get a COVID-19 shot, but they also recommend a booster dose.16 “Vaccination may occur in any trimester, and emphasis should be on vaccine receipt as soon as possible to maximize maternal and fetal health. This recommendation applies to both primary series and booster vaccination,” ACOG states.17
But missing from their information is data about natural immunity. Research by Makary and colleagues, published in JAMA,18 showed that natural immunity to COVID-19 — achieved by prior infection, not an injection — was durable for about two years, suggesting it’s longer lasting than any protection gained from a COVID-19 shot.19
A systematic review and meta-analysis published in The Lancet20 also revealed natural immunity to be at least as effective as, and likely superior to, COVID-19 injections.21 Health officials don’t have the data from Pfizer’s randomized trial of the shots in pregnant women. But rather than erring on the side of caution — especially for those with natural immunity — they’re still recommending everyone get the shots.
Scientists Warn Pregnant Women Not to Receive COVID Shots
Not everyone’s onboard with health agencies’ push for pregnant women to get injected. “The pushing of these experimental COVID-19 vaccines globally is the greatest violation of medical ethics in the history of medicine, maybe humanity,” Dr. James Thorp, a maternal fetal medicine expert, told Tucker Carlson.22
Thorp and colleagues published a preprint study that found striking risks to pregnant women who received the shots, along with their unborn babies.23 The outcomes were so dire that the researchers concluded pregnant women should not receive COVID-19 shots until further research is completed.
“A worldwide moratorium on the use of COVID-19 vaccines in pregnancy is advised until randomized prospective trials document safety in pregnancy and long-term follow-up in offspring,” they explained.24 Compared to the flu vaccine, COVID-19 shots were associated with a significant increase in adverse events (AE), including:25
Menstrual abnormality
Miscarriage
Fetal chromosomal abnormalities
Fetal malformation
Fetal cystic hygroma
Fetal cardiac disorders
Fetal arrhythmia
Fetal cardiac arrest
Fetal vascular mal-perfusion
Fetal growth abnormalities
Fetal abnormal surveillance
Fetal placental thrombosis
Low amniotic fluid
Fetal death/stillbirth
The data also revealed a 27-fold higher risk of miscarriage and a more than twofold increased risk of adverse fetal outcomes across six different categories, according to board-certified internist and cardiologist Dr. Peter McCullough.26
NIH Study Confirms Menstrual Changes Post-Jabs
Delayed menstruation has also been confirmed following COVID-19 shots, according to a study published in Obstetrics & Gynecology — funded by the National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health (NIH) Office of Research on Women's Health.27
The study involved 3,959 individuals between the ages of 18 and 45 years. Those who had not received a COVID-19 shot noted no significant changes in cycle four during the study compared to their first three cycles. But those who received COVID-19 shots had longer menstrual cycles, typically by less than one day, when they received the shots.
The European Union, meanwhile, has recommended that “heavy menstrual bleeding” be added as a side effect to mRNA COVID-19 shots.28 Makary noted:29
“Here in the U.S. there’s been no such update to product labeling. This lack of humility was also evident when healthy young women were told with incredible absolutism that the COVID vaccine cannot affect fertility. The right answer should have been: We don’t think it will affect fertility but we don’t have any good data on the question.”
Officials Choose ‘Stern Paternalism’ Over Informed Consent
In the absence of data for a vulnerable population — pregnant and breastfeeding women — health officials should have urged caution. As the effectiveness of natural immunity became clear, people should have been informed of this and warned of potential risks from COVID-19 shots. This way, they could make an informed decision before consenting to an injection that could have serious reproductive effects.
The Institute for Pure and Applied Knowledge (IPAK) felt the data were compelling enough in 2021 to withdraw the shots for vulnerable populations like pregnant women,30,31 but health officials chose, instead, to make them guinea pigs for an untested, experimental shot.
“In the absence of good data, organized medicine chose the path of stern paternalism. But in my experience as a physician, it’s far better to properly inform a patient rather than steamroll their questions,” said.32 If and when Pfizer does release the data, it’s possible that health agencies will have some explaining to do if the data aren’t favorable. Makary continued:33
“It may be that Pfizer’s pregnancy trial would have been favorable to the vaccine, showing that the benefits outweigh harms, but Pfizer has not released the data. Perhaps the data was not favorable, or perhaps Pfizer realized they had convinced the medical establishment without data, so why run the risk of sharing what a placebo-controlled trial shows?”
Sources and References
1, 5, 6, 7, 21, 29, 32, 33 Tablet June 21, 2023
2, 3, 13 U.S. CDC, COVID-19 Vaccines While Pregnant or Breastfeeding October 20, 2022
8, 10, 11 Substack, Maryanne Demasi reports February 23, 2023
16, 17 ACOG, COVID-19 Vaccines and Pregnancy: Conversation Guide
18 JAMA. 2022;327(11):1085-1087. doi: 10.1001/jama.2022.1393
23, 24, 25 Preprints 2022, 2022090430
27 Obstetrics & Gynecology: January 5, 2022 - Volume - Issue - 10.1097
30, 31 Science, Public Health Policy, and the Law Volume 4:130-143 November 2021
Source: The Daily Clout
Dr. McCullough Uncovers Smoking Gun: Autopsies Found 74% of deaths related to mRNA vaccination
July 14, 2023 • by The Wellness Company
The legacy of the COVID-19 pandemic and the widespread use of experimental vaccines continue to wreak havoc on the health of everyone on the planet, but now, there is frightening new data that suggests men are even more at risk.
Dr. Peter McCullough, Chief Scientific Officer at The Wellness Company, alongside his Chief Medical Board published an explosive scientific study in the prestigious scientific journal, The Lancet on July 6, 2023. The shockwaves are still being felt.
The study, titled A Systematic Review of Autopsy Findings in Deaths after COVID-19 Vaccination, exposed direct statistical evidence of our greatest fear: the COVID-19 vaccinations are a direct cause of DEATH, driving the excess mortality that we’ve witnessed after the government-pushed vaccination campaign.
From Dr. McCullough’s study: After surveying data from 325 autopsies, “a total of 270 deaths (73.9%) were independently adjudicated as directly due to or significantly contributed to by COVID-19 vaccination.” The review found that the primary organ system failure that caused death was the cardiovascular system (53% of cases). Says Dr. McCullough:
“Going forward in response to sudden unexplained deaths reported in the press, it is reasonable to conclude the cause of death is a fatal COVID-19 vaccine injury until proven otherwise.”
So, how do I get this out of my body?
Unfortunately, the story doesn’t end there. The study’s explosive findings ranked it as one of the most downloaded studies within 24 hours of publishing. Then, pathetically, the predictable happened: “prestigious” journal The Lancet removed the study from their website, claiming “the study’s conclusions are not supported by the study methodology.”
Bear in mind: the study is an analysis of ACTUAL 3rd PARTY AUTOPSY RESULTS. Therefore, The Lancet is effectively claiming that 270 independent autopsies are erroneous.
Because of these results, droves of people who were misled or coerced into taking the COVID-19 vaccination are now writing to Dr. McCullough and The Wellness Company, asking what can be done to mitigate their risk of death:
“Far and away the most common question I get from those who took one of the COVID-19 vaccines is: “how do I get this out of my body.” The mRNA and adenoviral DNA products were rolled out with no idea on how or when the body would ever breakdown the genetic code. The synthetic mRNA carried on lipid nanoparticles appears to be resistant to breakdown by human ribonucleases by design so the product would be long-lasting and produce the protein product of interest for a considerable time period…
This leaves dissolution of Spike protein as a therapeutic goal for the vaccine injured. With the respiratory infection, Spike is processed and activated by cellular proteases including transmembrane serine protein 2 (TMPRSS2), cathepsin, and furin. With vaccination, these systems may be avoided by systemic administration and production of Spike protein within cells. As a result, the pathogenesis of vaccine injury syndromes is believed to be driven by accumulation of Spike protein in cells, tissues, and organs.
Nattokinase is an enzyme is produced by fermenting soybeans with bacteria Bacillus subtilis var. natto and has been available as an oral supplement. It degrades fibrinogen, factor VII, cytokines, and factor VIII and has been studied for its cardiovascular benefits. Out of all the available therapies I have used in my practice and among all the proposed detoxification agents, I believe nattokinase and related peptides hold the greatest promise for patients at this time.”
Ways to connect
Telegram: @JoelWalbert
Email: thetruthaddict@tutanota.com
The Truth Addict Telegram channel
Hard Truth Soldier chat on Telegram
Mastodon: @thetruthaddict@noagendasocial.com
Session: 05e7fa1d9e7dcae8512eed0702531272de14a7f1e392591432551a336feb48357c
Odysee: TruthAddict
Donations (#Value4Value)
Buy Me a Coffee (One time donations as low as $1)
Bitcoin:
bc1qe8enf89g667dy890j2lnt637xqlt9wvc9f07un (on chain)
https://strike.me/nrn108
nemesis@getalby.com (lightning)
joelw@fountain.fm (lightning)
+wildviolet72C (PayNym)
Monero:
43E8i7Pzv1APDJJPEuNnQAV914RqzbNae15UKKurntVhbeTznmXr1P3GYzK9mMDnVR8C1fd8VRbzEf1iYuL3La3q7pcNmeN