Greetings fellow Plebs & Peasants,
If you think the word ‘placebo’ automatically means a saline injection or a sugar pill, think again. Often previously existing products with a well established record of advere events are used in order to blur the line of what is safe and what is not. A true inert placebo will not cause any adverse events (barring some already present serious underlying condition). For many years I could never understand how there were instances of people dying during trials from an inert placebo. It just made no sense whatsoever. Saline or sugar just will not kill a person. It wasn’t until late 2020 when having discussions with people about all the Covid injection trials deaths and laundry list of other serious events (the things we are now seeing en masse were in fact known prior to the EUA), that someone informed me that they are rarely if ever a true placebo. Suddenly it all made sense. So maybe you know this already, maybe you don’t. But what follows are analyses of what is really going on in these corrupt drug trials as far as the placebo scam goes.
#DoNotComply #NoCompromise #NoSurrender #GallowsAndGuillotines2023
- jw
Source: Health Freedom Defense Fund
“Safe and Effective” – Understanding Vaccine Clinical Trials
By HFDF Team
July 12, 2023
“Safe and effective,” the foundational creed of the vaccine orthodoxy, is enshrined by government health officials and medical professionals and is widely accepted by a largely under-informed public.
Aided by a compliant media, the vaccine industry itself relies on this mantra being accepted universally and unquestioningly.
Public health bureaucrats insist upon our obeisance to this medical catechism, demanding that we faithfully “follow the science and trust the experts.”
Yet, the specific methodologies used to determine the professed safety and efficacy of vaccines are little understood.
It is long past time to ask and answer: Are the testing protocols scientifically sound? Are the practices used in vaccine clinical trials trustworthy? How exactly is “safe and effective” determined?
We will examine the veracity of the “safe and effective” shibboleth by taking a critical look at one of the key elements of these testing procedure- the clinical trials themselves.
Randomized placebo-controlled clinical trials are considered, the “gold standard for evaluating the safety and efficacy of a new vaccine.”
The idea is to have participants in the trial randomized receive either the vaccine under investigation or a placebo.
How a placebo is defined and how the placebo is applied in practice are two points that, as we shall see, are crucial to the validity of any clinical trial.
The idea behind randomization and the use of a placebo is, “to control for confounding effects, such that significant differences in disease incidence or adverse effects between the vaccine and control groups can likely be attributed to the vaccine.”
What is a placebo?
The US Centers for Disease Control and Prevention (CDC) defines a placebo as, “a substance or treatment that has no effect on living beings, usually used as a comparison to vaccine or medicine in clinical trials.”
A placebo can be either an inert substance, such as a saline solution that is injected or a sugar pill that can be taken orally.
The importance of using a true placebo to get an accurate assessment of vaccine safety cannot be overstated.
In a 2018 letter, the legal team at the nonprofit Informed Consent Action Network (ICAN) challenged the US Department of Health and Human Services as to how HHS can justify,“licensing any pediatric vaccine without first conducting a long-term clinical trial in which the rate of adverse reactions is compared between the subject group and a control group receiving an inert placebo.”
Curiously, HHS defended its position, first by asserting that, “many pediatric vaccines have been investigated in clinical trials that included a placebo” [a false claim in and of itself] and then by adding, in the very next paragraph, “Inert placebo controls are not required to understand the safety profile of a new vaccine, and are thus not required.” [Emphasis added.]
This convoluted language not only raises questions about the consistency of the HHS’ position on placebos but it is patently absurd as it negates the possibility of knowing the true side-effects profile.
In that same paragraph, on page 2, HHS goes on to defend its position by claiming, “In cases where an active control is used, the adverse event profile of that control group is usually known and the findings of the study are reviewed in the context of that knowledge.”
This specious claim sidesteps the fact that knowing the adverse event profile of an active control group holds little meaning at best and is often outright deceptive in practice.
In an 88-page comprehensive follow-up to this dissembling, ICAN called into question the integrity of HHS:
The fact that HHS does not and apparently will not require pharmaceutical companies to use a placebo control in pediatric vaccine clinical trials evidences HHS’s lack of confidence in the safety profile of these products. If HHS had confidence in their safety profiles, it would require that vaccine clinical trials —– as is typical for drug clinical trials —– include a placebo-control group.
A clear example of this manipulation is illustrated by the clinical trial of Gardasil and its subsequent licensure.
In the Gardasil trial, 10,706 women received Gardasil; 9,092 women received Amorphous Aluminum Hydroxyphosphate Sulfate (AAHS), an “active control” used in the control group. Aluminum adjuvants, such as AAHS are known to induce autoimmunity in lab animals.
A small subset of participants, 320 women did receive a saline placebo. This smaller group was mixed in with the AAHS control group to form a “combined control group.”
In the six month study, 2.3% of the women receiving Gardasil and 2.3% of the women in the “combined control group” reported developing a systemic autoimmune disorder.
Based on these similar rates of systemic autoimmune disorders in both the “test group” and the “combined control group,” the vaccine was deemed safe and so was licensed by HHS.
What was not disclosed is that there were no autoimmune disorders among the 320 women who received the saline placebo. The reason this fact could be obfuscated was that the two control groups were combined.
Clearly, it is illogical, if not fraudulent, to claim a product is “safe” when it has an adverse event profile similar to an “active control” that has a poor adverse event profile.
Why was the safety profile of this product not directly compared to the women in the study who did use a genuine placebo?
Shouldn’t this information have triggered a larger follow-up study with a control group that used only the saline placebo?
The answer may lie in a 1998 article titled, “Drug Study Designs Guidance for Institutional Review Boards and Clinical Investigators,” in which the FDA points to issues that arise when active controls are used.
One of the problems the FDA cites is that there are, “numerous ways of conducting a study that can obscure differences between treatments adding that Active-control studies which are intended to show no significant difference between treatments.” [Emphasis added.]
Continues the FDA:
“In the absence of a placebo group, a finding of no difference in an active-control study therefore can mean that both agents are effective, that neither agent was effective in that study, or that the study was simply unable to tell effective from ineffective agents. In other words, to draw the conclusion that the test article was effective, one has to know with assurance that the active-control would have shown superior results to a placebo, had a placebo group been included in the study.” [Emphasis added.]
Using genuine placebos in safety studies is essential to demonstrate the true side effect profile of any drug. Absent a genuine placebo, it’s not possible to make precise claims about the actual risks of the drug being tested.
Clinical trials testing for efficacy that do not use a genuine placebo only tell us something about the efficacy of the product relative to the other product rather than the product’s absolute effectiveness. This allows for claims to be made that may be factually true but may misrepresent the overall impression.
So, when we see the word “placebo” mentioned in a study, it’s vital to ask
*“Does this mean that an inert substance was used in the study?”
* “Are the tests using real placebos or are they using placebos engineered to suit the objectives of the study?”
* “If a proper placebo is not being employed, will this lead to comparative distortions of adverse effects?”
To get an accurate picture of how placebos are defined and materially implemented in practice, we will explore three further examples of clinical trials that studied the safety and efficacy of the vaccines highlighted:
(1) In the largest of the clinical trials for GlaxoSmithKline’s (GSK) pediatric vaccine Pediarix, a 5-1 vaccine designed to protect against diphtheria, tetanus, pertussis, hepatitis B and polio, the placebo used by the control group was the pediatric DTaP vaccine Infanrix.
In 14 additional trials in that study (see page 8), the placebos received by the control groups are simply referred to as “comparator vaccines.”
Infanrix itself was tested using the older generation DTP vaccine (a vaccine known to cause serious side effects in infants) as the placebo for one control group in a clinical trial. No control group was used in another trial.
For Pediarix, no control group received a proper placebo.
(2) Our next example highlights Merck’s hepatitis ‘A’ vaccine VAQTA. The safety profile for this vaccine also did not include a true placebo control.
Neither of the two clinical trials for VAQTA used a proper control group. In the first trial, the “Monroe” study, it was acknowledged (see page 11) that the placebo utilized the aluminum adjuvant contained in the vaccine as well as thimerosal, a mercury- based neurotoxin phased out in the early 2000’s.
Neither of these substances can be considered inert or safe.
The second trial administered the vaccine alongside two other vaccines- called “historical control groups.”
This practice is widely considered unscientific, as it eliminates randomization. On page 62 of the Final Clinical Review (cited above) it is noted, Again, the use of historical controls is not the preferred trial design method.”
(3) Our third example is Merck’s VARIVAX, the first vaccine licensed for chicken pox. The safety section of the package insert for this vaccine stated that the product was tested using- “a double-blind, placebo-controlled study among 914 healthy children and adolescents who were serologically confirmed to be susceptible to varicella.”
Was this small study in fact “placebo-controlled” with a proper placebo?
As it turns out, the control group, for VARIVAX, was given a placebo described as being, “identical in appearance to the vaccine in both lyophilized and reconstituted forms, but contained no virus material. The placebo consisted of lyophilized stabilizer containing approximately 45 mg of neomycin per milliliter.”
In short, this meant that the control group was given the test vaccine minus the viral component. Perhaps this explains why the rates of adverse reactions were similar between the groups.
These three examples are not exceptional cases in the large body of literature on vaccine clinical trials. Sadly, this is standard operating procedure for virtually all vaccine trials.
The reality is that vaccines are generally or always tested either against an older form of the vaccine or against another vaccine or against a solution made of everything in the vaccine except the antigen in question.
In light of the emerging body of evidence, we have to ask ourselves:
“First do the current methods of testing provide a reliable measure of the safety and adverse effects of vaccines or do these methods serve to obfuscate potential harm?”
“Second, do the current methods of evaluation provide concrete evidence for the efficacy of these products?”
Beyond the matter of improper controls there are a host of additional confounding factors that are prevalent in vaccine trials. Problematic issues such as unblinding in trials, erratic clinical case definitions, biased statistics and design protocols, lack of long- term studies, lack of data on combinative impacts of multiple vaccines, and many additional questions materialize throughout these studies.
A critical reader of the scientific literature would grasp the implications of all of this and posit that the “safe and effective” bromide appears to be built on a foundation of quicksand.
Indeed, acceptance of this unassailable doctrine of the soundness of vaccines depends on the public not knowing the particulars of how these trials are conceived and carried out.
As an antidote to this information vacuum, HFDF will be presenting a comprehensive expose’ of the United States childhood vaccination schedule over the course of the next few weeks.
We shall look at each vaccine to see if in fact the science is sound, the studies are adequate, and the established dogma is credible.
Source: The Daily Skeptic
The Scam That Spins ‘95% Vaccine Efficacy’ From a Placebo
by Norman Fenton and Martin Neil
We have provided numerous explanations (see here, and here) and videos (see here and here) explaining why a vaccine that is actually merely a placebo will inevitably appear to have high efficacy if there is a time delay after vaccination during which the participant is classified as ‘unvaccinated’.
Some people have claimed that the examples – using hypothetical data – are unrealistic and that, with different assumptions about the underlying infection rate, the illusion would not happen. Not true.
This example simulates a vaccine roll-out and efficacy evaluation which is essentially how all the 2021 observational studies of the Covid vaccines were conducted.
Assumptions:
The entire population starts week 1 as unvaccinated and by week 14 about 90% of the population has received a single jab (note that we start with a population of one million but the efficacy results are exactly same irrespective of the starting population).
The vaccination rollout starts with 1% of the unvaccinated population vaccinated in week 1 and peaks at 35% in week 8 falling back to 10% in each of the last three weeks.
There is a constant weekly infection rate throughout the period (in the example below it is 2% but, as the video shows, the ‘efficacy’ numbers are exactly the same no matter what the fixed rate is).
Any vaccinated person who becomes infected within the first two weeks of his or her vaccination is classified as unvaccinated (in fact, as can be seen here, the Office for National Statistics classifies a person as infected within the first three weeks of his or her vaccination as unvaccinated. And also note this is the case in Sweden).
The Excel model can be downloaded from here.
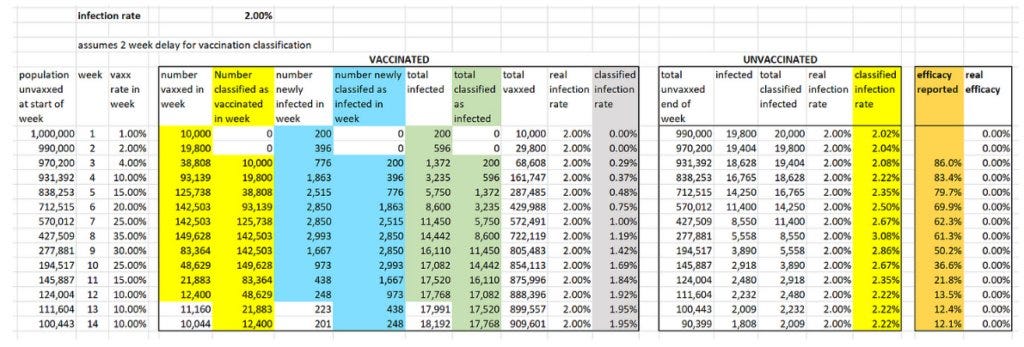
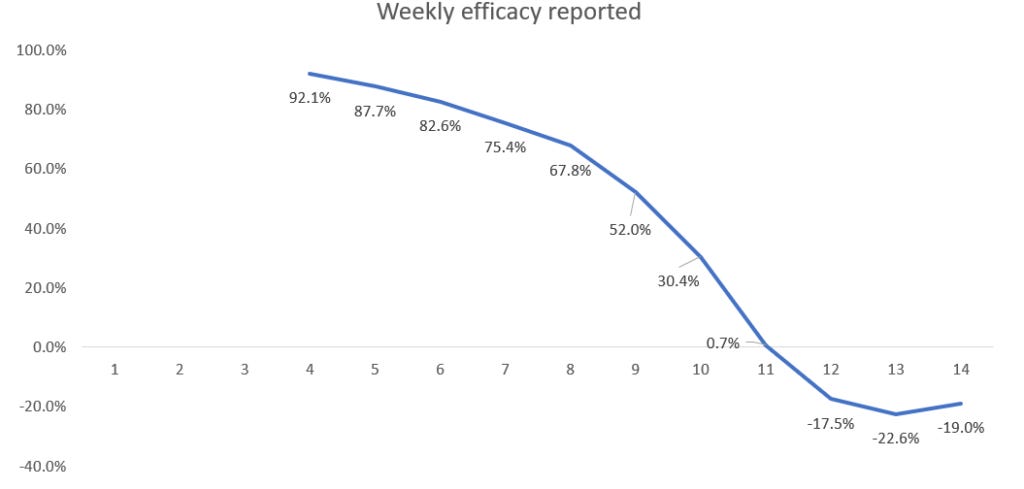
Based on these assumptions, we find that a placebo is 86% ‘effective’ under a 14-day rule.
Here are the results shown in our new video.
To calculate the infection rate for week n for those classified as vaccinated we divide the cumulative number of people classified as infected and vaccinated by week n by the cumulative number of people classified as vaccinated by end of week n.
For example, by end of week 4 a total of 161,147 people have been classified as vaccinated, of whom a total of 596 have been classified as infected. So the week 4 infection rate for the vaccinated is 596/161,147 which is 0.37%. We do the same for those classified as unvaccinated, so the week 4 infection rate for the unvaccinated is 2.22%. Note that, although the real weekly infection rates of the vaccinated and unvaccinated are always the same 2%, the infection rates after the ‘classification’ are always lower than 2% for those classified as ‘vaccinated’ and higher than 2% for those classified as ‘unvaccinated’.
To calculate the week n efficacy rate we divide the vaccinated infection rate by the unvaccinated infection rate and subtract this from 1, expressing the result as a percentage. Hence, the week 4 efficacy rate is 1 – (0.37÷2.22) = 0.834 = 83.4%.
Now we see that a completely useless (with true efficacy 0%) vaccine appears to have very high efficacy in the first few weeks. Although it continually wanes it is still above 50% after week 9. By week 14 the efficacy is still positive but only 12.1% – hence the need for a new booster dose! These simulated results are very similar to the real-world efficacy rates that were observed in the first three months of a new vaccine or booster.
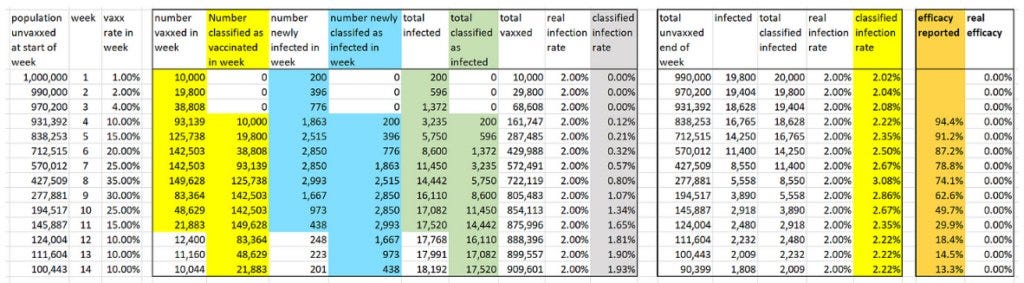
Here are the results if there is a three-week (21-day) period before a person is classified as vaccinated (as per ONS method).
The efficacy achievable under the three-week (21-day) period is 95%.
So now they could claim the vaccine starts with 95% efficacy, but again you need the booster after three months for vaccination to be truly effective.
For those who doubt the ONS calculates vaccine efficacy this way, here is a screenshot from its document.
The same applies for further doses where there is always a 21-day delay before being classified as having that latest booster shot.
Even negative efficacy can be made to look 95% effective.
Note that for a placebo vaccine it is impossible to get negative efficacy. However, if there is a slightly higher infection rate for the vaccine compared to no vaccine then the same assumptions as above still yield high efficacy initially (92% at week 4) before eventually becoming negative. Here are the results if the vaccine actually increases the infection rate by 50% (so a 3% infection rate in the vaccinated rather than the assumed 2% in the above simulations).
Some caveats. In practice there are a number of factors which, if we included them in the simulation, would produce an even higher efficacy rate than could be claimed for a placebo vaccine. For example:
The 2021 U.K. rollout happened during a period when the Covid infection rate was declining.
Asymptomatic unvaccinated people were much more likely to be required to take PCR tests (to go to work or attend events, restaurants etc.) than asymptomatic vaccinated people, since the latter only had to show their proof of vaccination. In Israel during this period an unvaccinated person was six times more likely to have to be PCR tested than a vaccinated person. The high false positive rate for asymptomatics would therefore artificially increase the infection rate of the unvaccinated.
People who were (or had recently been) PCR positive were not allowed to be vaccinated until 14 days after a negative test. This means the vaccinated cohort contained a higher proportion of people who already had natural immunity.
As we have shown the illusion of high efficacy is also present if the vaccine is worse than a placebo. In particular we know that, for the Covid vaccines, there was a disproportionately high infection rate within the first 14 days after vaccination. Given all of those infected within the first 14 days are classified as unvaccinated, this would lead to even higher efficacy rates than shown in our simulation.
On the issue of immunity, one simplification made in the simulation is that it does not take account of the fact that those who become infected during the period (whether vaccinated or not) would almost certainly not become infected again (and therefore should be removed from the count in subsequent weeks). However, unless the general infection rate is very high this has minimal impact on the efficacy results.
In conclusion, it may be reasonable to allow a certain amount of time for a vaccine to ‘work’. However, classifying a person who becomes infected within 14 or 21 days of vaccination as an ‘unvaccinated case’ in the calculation of vaccine efficacy is nothing short of a scam. It guarantees that any vaccine which is no different (or even worse) than a placebo will be seen to have high initial efficacy.
By using the 21-day period before considering a person vaccinated – as the ONS and others do for the Covid vaccines – means fraudulently high artificial efficacy rates are guaranteed. The apparent but completely artificial ‘waning’ of efficacy can also be used deviously to support the idea that after three or four months another dose of the vaccine is required to regain protection. Since the same delay in classification is used for those who have received a further dose, it is then guaranteed that high efficacy can again be claimed for the subsequent doses.
All of this creates a repeatable business model for Big Pharma.
Source: Nature of Healing
The False Placebo
By Rosanne Lindsay, Traditional Naturopath
July 6th, 2023
As much as we have tried to fuse science with medicine, much of the practice of medicine is not scientifically based. Much of what the physician does in practice is dictated by authority. – F.H.K. Green et al, Lancet, 1954
There is suddenly a lot of interest in people who may have received a placebo vaccine.
According to media reports, scientists have uncovered evidence that a large quantity of the Pfizer-BioNTech COVID-19 vaccine deployed in the European Union may in fact have consisted of placebos – and that the German regulator knew this and did not subject them to quality-control testing. The basis for this assertion is a March 2023 Danish study published in European Journal of Clinical Investigation.
However, the evidence in the Danish study is unclear. The results are based only on color coding of batches (yellow = harmless, blue = very bad, not so bad = green). What did the vials contain? Explain the demographics of the populations (age, sex)? What made up the placebos?
People who opted to get an experimental vaccine in the U.S. may rightly wonder if they, too, got a placebo or something else. A placebo is supposed to be harmless, used for experimental purposes to identify a legitimate baseline or control group.
Placebo definition: a harmless pill, medicine, or procedure prescribed more for the psychological benefit to the patient than for any physiological effect; used as a control in testing new drugs.
However, most people may not be aware that in “Vaccine Science” there is commonly no true placebo for control groups. Thus, there are no true control groups in vaccine trials. That means people receive a false placebo.
Why a false placebo? Because vaccine scientists want everyone to know that they care.
Vaccine Standard of Care
The World Health Organization (WHO) does not want to deprive anyone from receiving the benefit of any vaccine. That would be unethical, says WHO. So, according to the WHO’S Standard of Care, a placebo can be either:
an older version of the vaccine being tested, or
an adjuvant such as aluminum hydroxide, an ingredient common to all vaccines to increase a reaction (i.e, side effects).
The vaccine Standard of Care is common to most guidance documents. For instance, an August 2014 Journal Vaccine article titled, “Placebo use in vaccine trials: Recommendations of a WHO expert panel,” states:
…. randomised, placebo-controlled trial designs often raise ethical concerns when participants in the control arm are deprived of an existing vaccine. Furthermore, testing a new vaccine against placebo is scientifically and ethically fraught when the hypothesis being tested is whether an experimental vaccine is more efficacious than one already in use in the same or in other settings.
The False Placebo
The authority on vaccines, The World Health Organization (WHO), recommends using an older vaccine for saline in a placebo.
Meaning, if they are testing a new vaccine it would be unethical to test it against saline when an older, proven safe version exists. So, they can use the older version as the placebo and, therefore, not deprive the study participant of the protection. It is also considered ethical to use an adjuvant in lieu of a vaccine when the vaccine being studied has that adjuvant in it. So, you can use an aluminum adjuvant as a placebo if the adjuvant has been around enough to have been studied for safety.
An acceptable Standard of Care in vaccine trials means that the control group could receive either another vaccine, such as the Hep B vaccine, or an injection with an adjuvant (ie., aluminum hydroxide).
Dr. Christopher Exley studies and publishes on aluminum adjuvants in vaccines and their resulting cellular toxicity.
…paediatricians, responsible for administering the vaccine schedule for children, seem in particular, to be uninformed about the properties of aluminium adjuvants and their mode of action in vaccines. This apparent ignorance of the published scientific literature is unexpected in those charged with the wellbeing of neonates and infants and especially in the light of Janeway’s description of alum adjuvant as ‘the immunologist’s dirty little secret’ [2]
Aluminum adjuvants in vaccines are designed to remain in the body. There they trigger a cytokine storm that manifests a sequelae of symptoms known as Autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA). What does this mean?
The adjuvanted placebo is not a placebo at all.
Studies confirm that adjuvanted vaccines that claim to treat infectious disease lead to the development of autoimmune disease: Shoenfeld’s syndrome, Autoimmune thyroid disease, neurological disease, and longterm, chronic disease, Multiple Sclerosis, autism, Alzheimer’s disease.
The moral? To give a false placebo means that adverse events between the control and experimental groups in a study show little to no difference. The vaccine is declared “safe and effective.”
A Question of Ethics
The word ‘ethics’ is derived from the Greek word, ethos, which means custom or character. Ethics is the systematic study of values, so as to decide what is right and what is wrong.
However, the justification of clinical biomedical research on humans means definitions can change to suit authorities. In chapter 21 of The Oxford Textbook of Clinical Research Ethics, author Alan Wertheimer writes:
“The value of biomedical research is such that many commentators believe that society has a strong obligation to conduct, support, and otherwise encourage it.”
But what about an obligation to protect its members from harm? The author goes on to refer to a quote by Hans Jonas, professor of Philosophy:
Our descendants have a right to be left an unplundered planet; they do not have a right to new miracle cures. We have sinned against them if by our doing, we have destroyed their inheritance.
Medical doctors take The Hippocratic Oath to help, or at least to do no harm. But does the WHO’s authority supersede ethics?
Some in Congress are threatening to defund the authority, the WHO, as it seeks to implement a global pandemic treaty that replaces laws of nations. But is that possible when the U.S. president wants to support and strengthen the WHO?
History proves that the inhumane Tuskegee experiments occurred from 1932-1972, well after the Hippocratic Oath made its initial appearance around 400 BCE.
The evidence of Tuskegee shows how medical doctors do not always take the opportunity to help their patients get well. During Tuskegee, doctors knew how to successfully treat Syphilis, but instead chose to watch their patients suffer and die after lying about the diagnosis and giving a placebo instead of the standard treatment.
Following Tuskegee, in 1979, The Belmont Report set up guidelines and ethical principles. Then, in the year 2000, The Indian Council of Medical Research produced the ‘Ethical Guidelines for Biomedical Research on Human Subjects,’ which were revised in 2006. It gives twelve general principles to be followed by all biomedical researchers working in the country. The latest global guidelines are the International Ethical Guidelines for Biomedical Research Involving Human Subjects.
How many guidelines and principles are required to ensure no harm to human subjects?
Informed Consent
Informed consent is both an ethical and legal obligation. It is a doctor’s duty to explain and disclose the consequences of treatment and non-treatment. Thus, patients have the right of self-determination.
The Belmont Report stressed three basic ethical principles: 1) respect for person, 2) beneficence, and 3) justice. These were applied in the form of informed consent, assessment of risks and benefits by ethics committees and selection of subjects. However, today we know that patients are not provided full informed consent before agreeing to medical or experimental procedures.
Disclosure of information should include:
The condition/disorder/disease that the patient is having/suffering from
Necessity for further testing
Natural course of the condition and possible complications
Consequences of non-treatment
Treatment options available
Potential risks and benefits of treatment options
Duration and approximate cost of treatment
Expected outcome
Follow-up required
Ethics Vs. Morals
Currently, there is conflicting guidance on how to evaluate the use of placebo controls in vaccine trials.
Most ethical guidelines for research do not address vaccine trials specifically; and, in those that do, the guidance regarding placebo use is limited [2], [3].
Moreover, general ethical guidelines for research – authored by both national and international bodies – offer conflicting guidance on the use of placebo controls [4], [5], [6], [7], [8].
Some guidelines call for exclusion of placebo use altogether when there is a proven or established effective intervention against the condition under study.
The ethics of vaccine science asks why use a true placebo when testing a new product when you can substitute an older product?
However, the morals of vaccine science supersede ethical questions, or so they should.
Use of placebos in certain self-limiting conditions or in patients with high psychological overlay or in those who insist for some form of medication is justified as there are high chances of benefit to the patient with negligible risk. Revealing the truth to the patient takes away the very purpose of administration of placebo.
The authors of the Journal Vaccine study conclude:
The lack of consistent guidance on the use of placebo controls raises significant ethical concern… a lack of clear guidance may result in the conduct of placebo-controlled trials that are ultimately unethical.
Since the time of Hippocrates, human ethics has devolved. Regarding informed consent for any procedure, the question should not be whether you received a placebo, but rather, what is in the placebo?
Ways to connect
Telegram: @JoelWalbert
Email: thetruthaddict@tutanota.com
The Truth Addict Telegram channel
Hard Truth Soldier chat on Telegram
Mastodon: @thetruthaddict@noagendasocial.com
Session: 05e7fa1d9e7dcae8512eed0702531272de14a7f1e392591432551a336feb48357c
Odysee: TruthAddict
Donations (#Value4Value)
Buy Me a Coffee (One time donations as low as $1)
Bitcoin:
bc1qe8enf89g667dy890j2lnt637xqlt9wvc9f07un (on chain)
https://strike.me/nrn108
nemesis@getalby.com (lightning)
joelw@fountain.fm (lightning)
+wildviolet72C (PayNym)
Monero:
43E8i7Pzv1APDJJPEuNnQAV914RqzbNae15UKKurntVhbeTznmXr1P3GYzK9mMDnVR8C1fd8VRbzEf1iYuL3La3q7pcNmeN










Thank you! This is one of THE most important issues that doesn't get enough attention. I had a similar experience when I learned that they weren't really using placebo's in around 2017. Then to see the same thing so blatant during covid... I'm glad people are now realizing how critical this lie is.